Prevention of disc degeneration with growth factors
- PMID: 16865380
- PMCID: PMC2335371
- DOI: 10.1007/s00586-006-0149-1
Prevention of disc degeneration with growth factors
Abstract
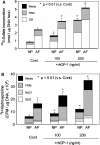
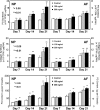
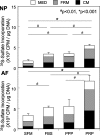
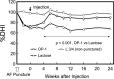
Clinically, a large number of patients have persistent low back pain attributable to intervertebral disc (IVD) degeneration. After the concept of biologically regenerating the degenerated IVD using growth factor injection was first proposed in early 1990, the advancement of molecular technology to produce recombinant proteins, including growth factors, on an industrial scale accelerated research in this field. The purpose of this review is to summarize the most recent findings of the in vitro and in vivo effects of growth factors on the IVD and, further, to discuss the limitations of growth factor therapy and its clinical implications. In vitro data showed that stimulation of matrix synthesis by growth factors alters the balance of homeostasis by shifting cellular metabolism to the anabolic state. In vivo data using small animals has shown the possibility of using growth factors as a "structural modifying therapy". Based on in vitro and in vivo data previously reported, the clinical application of growth factors by direct injection of protein into the nucleus pulposus or anulus fibrosus was shown to be feasible as a new therapeutic intervention for treatment of disc degeneration. Stimulation of the biological repair process will create a new category of therapy to treat disc degeneration, where no active treatment currently exists, between conservative therapies and more aggressive therapies such as fusion or disc replacement. However, it should be noted that there are several important factors to be taken into consideration. In a relatively advanced degenerative condition, the supply of nutrients is disturbed and stimulation of cellular activity by growth factors may result in an increased demand for nutrients, eventually inducing an adverse event. Further investigations of the optimal environment for growth factor stimulation should be pursued. Growth factor therapy, which has experimental evidence supporting it to be a "structural modifying therapy", may not be a "symptom modifying therapy" that is able to resolve the symptoms associated with pathologic changes. Therefore, further studies on the effect of growth factor therapy on pain are essential to shed light on its therapeutic usefulness for degenerative disc disease.
Figures

Similar articles
-
Biological repair of the degenerated intervertebral disc by the injection of growth factors.Eur Spine J. 2008 Dec;17 Suppl 4(Suppl 4):441-51. doi: 10.1007/s00586-008-0749-z. Epub 2008 Nov 13. Eur Spine J. 2008. PMID: 19005698 Free PMC article. Review.
-
Molecular therapy of the intervertebral disc.Eur Spine J. 2006 Aug;15 Suppl 3(Suppl 3):S379-88. doi: 10.1007/s00586-006-0155-3. Epub 2006 Jul 12. Eur Spine J. 2006. PMID: 16835736 Free PMC article. Review.
-
Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration.Spine (Phila Pa 1976). 2013 Jan 15;38(2):E49-58. doi: 10.1097/BRS.0b013e31827aa896. Spine (Phila Pa 1976). 2013. PMID: 23124260
-
Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc.J Pathol. 2005 Dec;207(4):445-52. doi: 10.1002/path.1862. J Pathol. 2005. PMID: 16278806
-
Tissue engineering of the intervertebral disc with cultured nucleus pulposus cells using atelocollagen scaffold and growth factors.Spine (Phila Pa 1976). 2012 Mar 15;37(6):452-8. doi: 10.1097/BRS.0b013e31823c8603. Spine (Phila Pa 1976). 2012. PMID: 22037529
Cited by
-
Unraveling the mechanisms of intervertebral disc degeneration: an exploration of the p38 MAPK signaling pathway.Front Cell Dev Biol. 2024 Jan 19;11:1324561. doi: 10.3389/fcell.2023.1324561. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38313000 Free PMC article. Review.
-
Is exclusion of leukocytes from platelet-rich plasma (PRP) a better choice for early intervertebral disc regeneration?Stem Cell Res Ther. 2018 Jul 18;9(1):199. doi: 10.1186/s13287-018-0937-7. Stem Cell Res Ther. 2018. PMID: 30021649 Free PMC article.
-
Platelet-Rich Plasma Releasate versus Corticosteroid for the Treatment of Discogenic Low Back Pain: A Double-Blind Randomized Controlled Trial.J Clin Med. 2022 Jan 7;11(2):304. doi: 10.3390/jcm11020304. J Clin Med. 2022. PMID: 35053999 Free PMC article.
-
Investigation of solute concentrations in a 3D model of intervertebral disc.Eur Spine J. 2009 Feb;18(2):254-62. doi: 10.1007/s00586-008-0822-7. Epub 2008 Nov 18. Eur Spine J. 2009. PMID: 19015897 Free PMC article.
-
Down-regulation of insulin-like growth factor binding protein 5 is involved in intervertebral disc degeneration via the ERK signalling pathway.J Cell Mol Med. 2019 Sep;23(9):6368-6377. doi: 10.1111/jcmm.14525. Epub 2019 Jul 10. J Cell Mol Med. 2019. PMID: 31290273 Free PMC article.
References
-
- Akeda K, An H, Pichika R, Attawia M, Lenz M, Uchida A, Thonar E, Masuda K. Platelet-rich Plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and annulus fibrosus cells cultured in alginate beads. Spine. 2006;31:959–966. doi: 10.1097/01.brs.0000214942.78119.24. - DOI - PubMed
-
- An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, Andersson GB, Masuda K. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

