Immunogenicity of recombinant hepatitis B vaccine in treatment-naive and treatment-experienced chronic hepatitis C patients: the effect of pegylated interferon plus ribavirin treatment
- PMID: 16865790
- PMCID: PMC4087759
- DOI: 10.3748/wjg.v12.i27.4420
Immunogenicity of recombinant hepatitis B vaccine in treatment-naive and treatment-experienced chronic hepatitis C patients: the effect of pegylated interferon plus ribavirin treatment
Abstract
Aim: To retrospectively evaluate the vaccination-induced anti-HBs seroconversion rates in treatment-naive and treatment-experienced chronic hepatitis C (CHC) patients. Also to prospectively evaluate the seroconversion rates in CHC patients during pegylated interferon (PEG) plus ribavirin (RIB) treatment.
Methods: Seventy treatment-naive CHC patients (group A), 22 sustained virological responders-SVR following interferon (IFN) plus RIB treatment CHC patients (group B) and 121 healthy subjects (group C) had been participated in the same HBV vaccination schedule (20 microg, 0-1-6 mo). Seroconversion was considered if anti-HBs levels were above 10 mIU/mL within 3 mo following the third dose of the vaccine. Moreover, we prospectively selected 30 non-cirrhotic CHC patients and evaluated them for the efficacy of the same vaccine schedule randomizing them in two groups: Group-1, 15 CHC patients received the first dose of the vaccine in parallel with the initiation of PEG plus RIB treatment and Group-2, 15 patients received the same vaccination schedule without concomitant treatment. Determination of anti-HBs was performed at mo 1, 2, and 7. Statistical analysis of data was based on ANOVA student's t-test and chi-square analysis (P < 0.05).
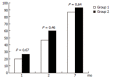
Results: Fifty-eight of 70 group A patients (82.85%), 20/22 group B (90.9%) and 112/121 healthy subjects (92.56%) had been seroconverted. The seroconversion rates were significantly higher in the control group than in treatment-naive CHC patients (P = 0.04). The corresponding rates were comparable between group A and group B CHC patients (P = 0.38). The vast majority of non-responders (10/14, 71.43%) had been infected by genotype-1 of HCV. The seroconversion rates were comparable between group 1 and 2 CHC patients at mo 1 (20% versus 26.7%, P = 0.67), mo 2 (46.7% vs 60%, P = 0.46) and mo 7 (86.7% versus 93.3%, P = 0.54) of follow-up.
Conclusion: The immunogenicity of HBV vaccine seems to be lower in CHC patients compared to healthy subjects. SVR following IFN plus RIB treatment does not affect the antibody response to HBV vaccine. Infection by genotype-1 seems to negatively influence the seroconversion rates. Vaccination against HBV during PEG plus RIB combination treatment is not beneficial in terms of anti-HBs seroconversion rates.
Figures
Similar articles
-
Therapeutic effects of pegylated interferon plus ribavirin in chronic hepatitis C patients with occult hepatitis B virus dual infection.J Gastroenterol Hepatol. 2010 Feb;25(2):259-63. doi: 10.1111/j.1440-1746.2009.06006.x. Epub 2009 Oct 9. J Gastroenterol Hepatol. 2010. PMID: 19817959
-
Sustained HCV-RNA response and hepatitis Bs seroconversion after individualized antiviral therapy with pegylated interferon alpha plus ribavirin and active vaccination in a hepatitis C virus/hepatitis B virus-coinfected patient.Eur J Gastroenterol Hepatol. 2007 Oct;19(10):906-9. doi: 10.1097/MEG.0b013e3282094160. Eur J Gastroenterol Hepatol. 2007. PMID: 17873617
-
Maintenance of T1 response as induced during PEG-IFNalpha plus ribavirin therapy controls viral replication in genotype-1 patients with chronic hepatitis C.Rev Esp Enferm Dig. 2005 Jul;97(7):481-90. doi: 10.4321/s1130-01082005000700003. Rev Esp Enferm Dig. 2005. PMID: 16262527 English, Spanish.
-
Hepatitis C: viral and host factors associated with non-response to pegylated interferon plus ribavirin.Liver Int. 2010 Oct;30(9):1259-69. doi: 10.1111/j.1478-3231.2010.02283.x. Liver Int. 2010. PMID: 20633102 Free PMC article. Review.
-
Treatment with peginterferon plus ribavirin vs. interferon plus ribavirin for 48 weeks in Chinese patients with chronic hepatitis C.Int J Clin Pract. 2009 Sep;63(9):1334-9. doi: 10.1111/j.1742-1241.2009.02082.x. Int J Clin Pract. 2009. PMID: 19691617 Review.
Cited by
-
Humoral Immune Response after COVID-19 mRNA Vaccination in Patients with Liver Cirrhosis: A Prospective Real-Life Single Center Study.Biomedicines. 2023 Apr 28;11(5):1320. doi: 10.3390/biomedicines11051320. Biomedicines. 2023. PMID: 37238990 Free PMC article.
-
Response to hepatitis B virus vaccination in individuals with chronic hepatitis C virus infection.PLoS One. 2020 Aug 26;15(8):e0237398. doi: 10.1371/journal.pone.0237398. eCollection 2020. PLoS One. 2020. PMID: 32845914 Free PMC article.
-
Chronic bystander infections and immunity to unrelated antigens.Cell Host Microbe. 2012 Oct 18;12(4):458-69. doi: 10.1016/j.chom.2012.10.001. Cell Host Microbe. 2012. PMID: 23084915 Free PMC article. Review.
-
Bloodborne viral hepatitis infections among drug users: the role of vaccination.Int J Environ Res Public Health. 2009 Jan;6(1):400-13. doi: 10.3390/ijerph6010400. Epub 2009 Jan 22. Int J Environ Res Public Health. 2009. PMID: 19440291 Free PMC article. Review.
-
Hepatitis A and hepatitis B vaccination responses in persons with chronic hepatitis C infections: A review of the evidence and current recommendations.Can J Infect Dis Med Microbiol. 2008 Mar;19(2):197-202. doi: 10.1155/2008/410362. Can J Infect Dis Med Microbiol. 2008. PMID: 19352452 Free PMC article.
References
-
- Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. - PubMed
-
- Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. - PubMed
-
- Delage G, Infante-Rivard C, Chiavetta JA, Willems B, Pi D, Fast M. Risk factors for acquisition of hepatitis C virus infection in blood donors: results of a case-control study. Gastroenterology. 1999;116:893–899. - PubMed
-
- Lemon SM, Thomas DL. Vaccines to prevent viral hepatitis. N Engl J Med. 1997;336:196–204. - PubMed
-
- Encke J, zu Putlitz J, Wands JR. DNA vaccines. Intervirology. 1999;42:117–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources