Activity of the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid against human head and neck carcinoma xenografts
- PMID: 16867215
- PMCID: PMC1601938
- DOI: 10.1593/neo.06295
Activity of the vascular-disrupting agent 5,6-dimethylxanthenone-4-acetic acid against human head and neck carcinoma xenografts
Abstract
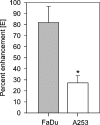
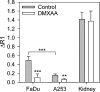
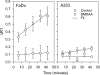
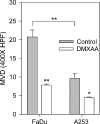
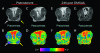
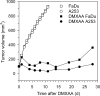
Head and neck squamous cell carcinomas (HNSCC) constitute a majority of the tumors of the upper aerodigestive tract and continue to present a significant therapeutic challenge. To explore the potential of vascular-targeted therapy in HNSCC, we investigated the antivascular, antitumor activity of the potent vascular-disrupting agent (VDA) 5,6-dimethylxanthenone-4-acetic acid (DMXAA) against two HNSCC xenografts with markedly different morphologic and vascular characteristics. Athymic nude mice bearing subcutaneous FaDu (human pharyngeal squamous cell carcinoma) and A253 (human submaxillary gland epidermoid carcinoma) tumors were administered a single dose of DMXAA (30 mg/kg, i.p). Changes in vascular function were evaluated 24 hours after treatment using contrast-enhanced magnetic resonance imaging (MRI) and immunohistochemistry (CD31). Signal enhancement (E) and change in longitudinal relaxation rates (deltaR1) were calculated to measure alterations in vascular perfusion. MRI showed a 78% and 49% reduction in vascular perfusion in FaDu and A253 xenografts, respectively. CD31-immunostaining of tumor sections revealed three-fold (FaDu) and two-fold (A253) reductions in microvessel density (MVD) 24 hours after treatment. DMXAA was equally effective against both xenografts, with significant tumor growth inhibition observed 30 days after treatment. These results indicate that DMXAA may be clinically beneficial in the management of head and neck cancers, alone or in combination with other treatments.
Figures

Similar articles
-
Tumor vascular response to photodynamic therapy and the antivascular agent 5,6-dimethylxanthenone-4-acetic acid: implications for combination therapy.Clin Cancer Res. 2005 Jun 1;11(11):4241-50. doi: 10.1158/1078-0432.CCR-04-2703. Clin Cancer Res. 2005. PMID: 15930363
-
Monitoring antivascular therapy in head and neck cancer xenografts using contrast-enhanced MR and US imaging.Angiogenesis. 2011 Dec;14(4):491-501. doi: 10.1007/s10456-011-9233-1. Epub 2011 Sep 7. Angiogenesis. 2011. PMID: 21901534 Free PMC article.
-
Vascular disruption in combination with mTOR inhibition in renal cell carcinoma.Mol Cancer Ther. 2012 Feb;11(2):383-92. doi: 10.1158/1535-7163.MCT-11-0748. Epub 2011 Nov 14. Mol Cancer Ther. 2012. PMID: 22084164 Free PMC article.
-
Tumor dose response to the vascular disrupting agent, 5,6-dimethylxanthenone-4-acetic acid, using in vivo magnetic resonance spectroscopy.Clin Cancer Res. 2005 May 15;11(10):3705-13. doi: 10.1158/1078-0432.CCR-04-2504. Clin Cancer Res. 2005. PMID: 15897567
-
Temporal aspects of the action of ASA404 (vadimezan; DMXAA).Expert Opin Investig Drugs. 2010 Nov;19(11):1413-25. doi: 10.1517/13543784.2010.529128. Expert Opin Investig Drugs. 2010. PMID: 20964495 Free PMC article. Review.
Cited by
-
Radiotherapy and the cellular DNA damage response: current and future perspectives on head and neck cancer treatment.Cancer Drug Resist. 2020 Sep 17;3(4):775-790. doi: 10.20517/cdr.2020.49. eCollection 2020. Cancer Drug Resist. 2020. PMID: 35582232 Free PMC article. Review.
-
Neoplasia: the second decade.Neoplasia. 2008 Dec;10(12):1314-24. doi: 10.1593/neo.81372. Neoplasia. 2008. PMID: 19048110 Free PMC article.
-
The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents.Cancer Treat Rev. 2011 Feb;37(1):63-74. doi: 10.1016/j.ctrv.2010.05.001. Epub 2010 Jun 8. Cancer Treat Rev. 2011. PMID: 20570444 Free PMC article. Review.
-
Dll4 blockade potentiates the anti-tumor effects of VEGF inhibition in renal cell carcinoma patient-derived xenografts.PLoS One. 2014 Nov 13;9(11):e112371. doi: 10.1371/journal.pone.0112371. eCollection 2014. PLoS One. 2014. PMID: 25393540 Free PMC article.
-
Tumor vascular maturation and improved drug delivery induced by methylselenocysteine leads to therapeutic synergy with anticancer drugs.Clin Cancer Res. 2008 Jun 15;14(12):3926-32. doi: 10.1158/1078-0432.CCR-08-0212. Clin Cancer Res. 2008. PMID: 18559614 Free PMC article.
References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol. 2004;22:1743–1752. - PubMed
-
- Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortes-Funes H, Hitt R, Gascon P, Amellal N, Harstrick A, Eckardt A. Phase II multi-center study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5568–5577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
