Loss of adhesion in the circulation converts amelanotic metastatic melanoma cells to melanotic by inhibition of AKT
- PMID: 16867216
- PMCID: PMC1601939
- DOI: 10.1593/neo.05655
Loss of adhesion in the circulation converts amelanotic metastatic melanoma cells to melanotic by inhibition of AKT
Abstract
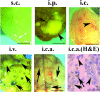
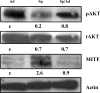
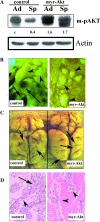
Direct injection of murine K-1735 melanoma cells into the subcutis, lung, or brain of syngeneic mice produces amelanotic tumors, whereas intravenous injection into the lateral tail vein or internal carotid artery produces both amelanotic and melanotic foci in the lung and the brain respectively. We hypothesized that loss of adhesion in the circulation may contribute to the melanogenic phenotypes of cells. To test this, we used enforced suspension culture of K-1735 cells by consistent rotating culture of K-1735 cells. We found that the expression of the microphthalmia transcription factor (MITF) and melanin-stimulating hormone receptor (MSHR) were upregulated in cells growing in suspension and were accompanied by inhibitions of AKT and ERK, which were reversed in cells upon regrowth as an adherent monolayer. Inhibition of the AKT pathway was responsible for MITF induction by suspension culture. Stable expression of constitutively active AKT significantly repressed the melanogenesis of K-1735 cells injected via circulation. An amelanotic clone of K-1735 cells was resistant to suspension culture-induced MITF, although the inhibition of AKT pathway was intact. Collectively, these data suggest that the inhibition of AKT pathway due to loss of adhesion within the circulation renders a subpopulation of K-1735 cells to produce melanin.
Figures


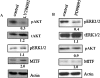

Similar articles
-
Transcriptional induction of the melanocyte-stimulating hormone receptor in brain metastases of murine K-1735 melanoma.Cancer Res. 1995 Jan 1;55(1):141-8. Cancer Res. 1995. PMID: 7805024
-
Comparative decreases in tyrosinase, TRP-1, TRP-2, and Pmel 17/silver antigenic proteins from melanotic to amelanotic stages of syngeneic mouse cutaneous melanomas and metastases.Cancer Res. 1998 Apr 1;58(7):1521-3. Cancer Res. 1998. PMID: 9537258
-
The role of 8q24 copy number gains and c-MYC expression in amelanotic cutaneous melanoma.Mod Pathol. 2012 Sep;25(9):1221-6. doi: 10.1038/modpathol.2012.75. Epub 2012 May 4. Mod Pathol. 2012. PMID: 22555175
-
Dichloromethane fraction of Cimicifuga heracleifolia decreases the level of melanin synthesis by activating the ERK or AKT signaling pathway in B16F10 cells.Exp Dermatol. 2009 Mar;18(3):232-7. doi: 10.1111/j.1600-0625.2008.00794.x. Epub 2008 Sep 18. Exp Dermatol. 2009. PMID: 18803655
-
Baicalin-induced Akt activation decreases melanogenesis through downregulation of microphthalmia-associated transcription factor and tyrosinase.Eur J Pharmacol. 2015 Aug 15;761:19-27. doi: 10.1016/j.ejphar.2015.04.028. Epub 2015 Apr 29. Eur J Pharmacol. 2015. PMID: 25934572
Cited by
-
Inhibitory effects of Taraxacum mongolicum with phreatic water on melanin synthesis.Integr Med Res. 2015 Jun;4(2):76-93. doi: 10.1016/j.imr.2014.08.001. Epub 2014 Aug 30. Integr Med Res. 2015. PMID: 28664113 Free PMC article.
-
Neoplasia: the second decade.Neoplasia. 2008 Dec;10(12):1314-24. doi: 10.1593/neo.81372. Neoplasia. 2008. PMID: 19048110 Free PMC article.
-
Expression of human papilloma virus type 16 E5 protein in amelanotic melanoma cells regulates endo-cellular pH and restores tyrosinase activity.J Exp Clin Cancer Res. 2009 Jan 8;28(1):4. doi: 10.1186/1756-9966-28-4. J Exp Clin Cancer Res. 2009. PMID: 19133143 Free PMC article.
-
p16INK4a expression and absence of activated B-RAF are independent predictors of chemosensitivity in melanoma tumors.Neoplasia. 2008 Nov;10(11):1231-9. doi: 10.1593/neo.08702. Neoplasia. 2008. PMID: 18953432 Free PMC article.
-
Reprogramming human A375 amelanotic melanoma cells by catalase overexpression: Reversion or promotion of malignancy by inducing melanogenesis or metastasis.Oncotarget. 2016 Jul 5;7(27):41142-41153. doi: 10.18632/oncotarget.9220. Oncotarget. 2016. PMID: 27206672 Free PMC article.
References
-
- Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. - PubMed
-
- Price JE, Tarin D, Fidler IJ. Influence of organ microenvironment on pigmentation of a metastatic murine melanoma. Cancer Res. 1988;48:2258–2264. - PubMed
-
- Schackert G, Fidler IJ. Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 1998;48:3478–3483. - PubMed
-
- Fujimaki T, Price JE, Fan D, Bucana C. Selective growth of human melanoma cells in the brain parenchyma of nude mice. Melanoma Res. 1996;6(5):363–371. - PubMed
-
- Fidler IJ, Gruys E, Cifone MA, Barnes Z, Bucana C. Demonstration of multiple phenotypic diversities in a murine melanoma of recent origin. J Natl Cancer Inst. 1981;67:947–956. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
