AP-2gamma induces p21 expression, arrests cell cycle, and inhibits the tumor growth of human carcinoma cells
- PMID: 16867219
- PMCID: PMC1601932
- DOI: 10.1593/neo.06367
AP-2gamma induces p21 expression, arrests cell cycle, and inhibits the tumor growth of human carcinoma cells
Abstract
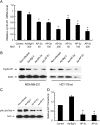
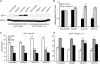
Activating enhancer-binding protein 2gamma (AP-2gamma) is a member of the developmentally regulated AP-2 transcription factor family that regulates the expression of many downstream genes. Whereas the effects of AP-2alpha overexpression on cell growth are fairly well established, the cellular effects of AP-2gamma overexpression are less well studied. Our new findings show that AP-2gamma significantly upregulates p21 mRNA and proteins, inhibits cell growth, and decreases clonogenic survival. Cell cycle analysis revealed that forced AP-2gamma expression induced G1-phase arrest, decreased DNA synthesis, and decreased the fraction of cells in S phase. AP-2gamma expression also led to cyclin D1 repression, decreased Rb phosphorylation, and decreased E2F activity in breast carcinoma cells. AP-2gamma binding to the p21 promoter was observed in vivo, and the absence of growth inhibition in response to AP-2gamma expression in p21(-/-) cells demonstrated that p21 caused, at least in part, AP-2-induced cell cycle arrest. Finally, the tumor growth of human breast carcinoma cells in vivo was inhibited by the expression of AP-2gamma relative to empty vector-infected cells, suggesting that AP-2gamma acts as a tumor suppressor. In summary, expression of either AP-2gamma or AP-2alpha inhibited breast carcinoma cell growth; thus, these genes may be therapeutic targets for breast cancer.
Figures





Similar articles
-
AP-2alpha and AP-2gamma are transcriptional targets of p53 in human breast carcinoma cells.Oncogene. 2006 Aug 31;25(39):5405-15. doi: 10.1038/sj.onc.1209534. Epub 2006 Apr 24. Oncogene. 2006. PMID: 16636674
-
The AP-1 transcription factor regulates breast cancer cell growth via cyclins and E2F factors.Oncogene. 2008 Jan 10;27(3):366-77. doi: 10.1038/sj.onc.1210643. Epub 2007 Jul 16. Oncogene. 2008. PMID: 17637753
-
Bone morphogenetic protein-2 induces hypophosphorylation of Rb protein and repression of E2F in androgen-treated LNCaP human prostate cancer cells.Int J Mol Med. 2005 Feb;15(2):253-8. Int J Mol Med. 2005. PMID: 15647840
-
The biological characteristics of transcription factors AP-2α and AP-2γ and their importance in various types of cancers.Biosci Rep. 2019 Mar 15;39(3):BSR20181928. doi: 10.1042/BSR20181928. Print 2019 Mar 29. Biosci Rep. 2019. PMID: 30824562 Free PMC article. Review.
-
[Dual role of transcription factor AP-2 in carcinogenesis].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010 Jul;39(4):430-5. doi: 10.3785/j.issn.1008-9292.2010.04.017. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 20731046 Review. Chinese.
Cited by
-
Aurora-A interacts with AP-2α and down regulates its transcription activity.PLoS One. 2011;6(8):e23110. doi: 10.1371/journal.pone.0023110. Epub 2011 Aug 1. PLoS One. 2011. PMID: 21829699 Free PMC article.
-
AP2gamma regulates basal progenitor fate in a region- and layer-specific manner in the developing cortex.Nat Neurosci. 2009 Oct;12(10):1229-37. doi: 10.1038/nn.2399. Epub 2009 Sep 13. Nat Neurosci. 2009. PMID: 19749747
-
Methylator phenotype of malignant germ cell tumours in children identifies strong candidates for chemotherapy resistance.Br J Cancer. 2011 Aug 9;105(4):575-85. doi: 10.1038/bjc.2011.218. Epub 2011 Jun 28. Br J Cancer. 2011. PMID: 21712824 Free PMC article.
-
Curcumin inhibits AP-2γ-induced apoptosis in the human malignant testicular germ cells in vitro.Acta Pharmacol Sin. 2013 Sep;34(9):1192-200. doi: 10.1038/aps.2013.38. Epub 2013 May 20. Acta Pharmacol Sin. 2013. PMID: 23685957 Free PMC article.
-
Nicotine induces EP4 receptor expression in lung carcinoma cells by acting on AP-2α: The intersection between cholinergic and prostanoid signaling.Oncotarget. 2017 May 19;8(44):75854-75863. doi: 10.18632/oncotarget.18023. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100274 Free PMC article.
References
-
- Mitchell PJ, Wang C, Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40T antigen. Cell. 1987;50:847–861. - PubMed
-
- Williams T, Admon A, Luscher B, Tjian R. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 1988;2:1557–1569. - PubMed
-
- Moser M, Imhof A, Pscherer A, Bauer R, Amselgruber W, Sinowatz F, Hofstadter F, Schule R, Buettner R. Cloning and characterization of a second AP-2 transcription factor: AP-2 beta. Development. 1995;121:2779–2788. - PubMed
-
- Chazaud C, Oulad-Abdelghani M, Bouillet P, Decimo D, Chambon P, Dolle P. AP-2.2, a novel gene related to AP-2, is expressed in the forebrain, limbs and face during mouse embryogenesis. Mech Dev. 1996;54:83–94. - PubMed
-
- Zhao F, Satoda M, Licht JD, Hayashizaki Y, Gelb BD. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J Biol Chem. 2001;276:40755–40760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
