Conserved features of germination and polarized cell growth: a few insights from a pollen-fern spore comparison
- PMID: 16867999
- PMCID: PMC2802967
- DOI: 10.1093/aob/mcl159
Conserved features of germination and polarized cell growth: a few insights from a pollen-fern spore comparison
Abstract
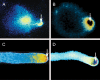
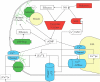
Background: The germination of both pollen and fern spores results in the emergence of a cell-pollen tube from pollen, rhizoid from spore-that grows in a polar fashion, primarily at its apical end. In both of these tip-growing cells, the delivery of secretory vesicles to the growing end is guided in part by a calcium gradient, with calcium entering at the tip where it is most highly concentrated. The similarities between the two systems extend beyond tip-focused calcium gradients to encompass signalling pathways and elements including calmodulin, nitric oxide, annexins and Rop-GTPases.
Scope and aims: This review is limited to those pathways and elements that function similarly in fern and pollen systems based on currently available evidence. The aim is to illustrate the common mechanisms by which tip growth occurs, facilitate further investigations into this area, and examine the implications for the evolutionarily conserved control of tip growth.
Conclusions: The interplay of calcium, nitric oxide and other effectors in both pollen and fern spores suggests certain signalling pathways became important regulators of germination and growth early in the evolution of land plants. Both large- and small-scale comparative genomic methods have shown to be promising in their ability to find new and relevant comparisons for further research. Cross-species comparisons may serve to speed up this process by highlighting both basic pathways and system-specific deviations.
Figures
Similar articles
-
Intersection of two signalling pathways: extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide.J Exp Bot. 2009;60(7):2129-38. doi: 10.1093/jxb/erp091. Epub 2009 Apr 10. J Exp Bot. 2009. PMID: 19363208 Free PMC article.
-
Glycolysis regulates pollen tube polarity via Rho GTPase signaling.PLoS Genet. 2018 Apr 27;14(4):e1007373. doi: 10.1371/journal.pgen.1007373. eCollection 2018 Apr. PLoS Genet. 2018. PMID: 29702701 Free PMC article.
-
Cytological and Proteomic Analyses of Osmunda cinnamomea Germinating Spores Reveal Characteristics of Fern Spore Germination and Rhizoid Tip Growth.Mol Cell Proteomics. 2015 Sep;14(9):2510-34. doi: 10.1074/mcp.M114.047225. Epub 2015 Jun 19. Mol Cell Proteomics. 2015. PMID: 26091698 Free PMC article.
-
Using maize as a model to study pollen tube growth and guidance, cross-incompatibility and sperm delivery in grasses.Ann Bot. 2011 Sep;108(4):727-37. doi: 10.1093/aob/mcr017. Epub 2011 Feb 23. Ann Bot. 2011. PMID: 21345919 Free PMC article. Review.
-
Calcium - a central regulator of pollen germination and tube growth.Biochim Biophys Acta. 2013 Jul;1833(7):1573-81. doi: 10.1016/j.bbamcr.2012.10.009. Epub 2012 Oct 13. Biochim Biophys Acta. 2013. PMID: 23072967 Review.
Cited by
-
Transcriptomic Insight into the Pollen Tube Growth of Olea europaea L. subsp. europaea Reveals Reprogramming and Pollen-Specific Genes Including New Transcription Factors.Plants (Basel). 2023 Aug 8;12(16):2894. doi: 10.3390/plants12162894. Plants (Basel). 2023. PMID: 37631106 Free PMC article.
-
Gene expression changes induced by space flight in single-cells of the fern Ceratopteris richardii.Planta. 2008 Dec;229(1):151-9. doi: 10.1007/s00425-008-0817-y. Epub 2008 Sep 20. Planta. 2008. PMID: 18807069
-
An osmotic model of the growing pollen tube.PLoS One. 2012;7(5):e36585. doi: 10.1371/journal.pone.0036585. Epub 2012 May 16. PLoS One. 2012. PMID: 22615784 Free PMC article.
-
Hypergravity prevents seed production in Arabidopsis by disrupting pollen tube growth.Planta. 2009 Oct;230(5):863-70. doi: 10.1007/s00425-009-0992-5. Epub 2009 Aug 1. Planta. 2009. PMID: 19649651
-
Pollen-tube tip growth requires a balance of lateral propagation and global inhibition of Rho-family GTPase activity.J Cell Sci. 2010 Feb 1;123(Pt 3):340-50. doi: 10.1242/jcs.039180. Epub 2010 Jan 5. J Cell Sci. 2010. PMID: 20053639 Free PMC article.
References
-
- Alessa L, Kropf DL. F–actin marks the rhizoid pole in living Pelvetia compressa zygotes. Development. 1999;126:201–209. - PubMed
-
- Battey NH, Blackbourn HD. The control of exocytosis in plant cells. New Phytologist. 1993;125:307–338. - PubMed
-
- Bednarska E. The effect of exogenous Ca2+ ions on pollen grain germination and pollen tube growth: investigations with 45Ca2+ together with verapamil, La3+ and ruthenium red. Sexual Plant Reproduction. 1989;2:53–58.

