Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy
- PMID: 16868252
- PMCID: PMC1895424
- DOI: 10.1182/blood-2006-04-017913
Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy
Abstract
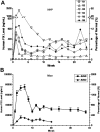
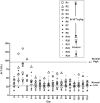
In a clinical study of recombinant adeno-associated virus-2 expressing human factor IX (AAV2-FIX), we detected 2 impediments to long-term gene transfer. First, preexisting anti-AAV neutralizing antibodies (NABs) prevent vector from reaching the target tissue, and second, CD8(+) T-cell responses to hepatocyte-cell surface displayed AAV-capsid-terminated FIX expression after several weeks. Because the vector is incapable of synthesizing viral proteins, a short course of immunosuppression, until AAV capsid is cleared from the transduced cells, may mitigate the host T-cell response, allowing long-term expression of FIX. To evaluate coad-ministration of immunosuppression, we studied AAV8 vector infusion in rhesus macaques, natural hosts for AAV8. We administered AAV8-FIX in 16 macaques via the hepatic artery and assessed the effects of (1) preexisting anti-AAV8 NABs, (2) a standard T-cell immunosuppressive regimen, and (3) efficacy and safety of AAV8-FIX. We found that low titers (1:5) of preexisting NABs abrogate transduction, whereas animals with undetectable NABs are safely and effectively transduced by AAV8-FIX. Coadministration of mycophenolate mofetil and tacrolimus with vector does not induce toxicity and does not impair AAV transduction or FIX synthesis. These findings enable a clinical study to assess the effects of immunomodulation on long-term FIX expression in patients with hemophilia B.
Figures


References
-
- Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99: 2670-2676. - PubMed
-
- Jiang H, Lillicrap D, Patarroyo-White S, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6 and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108: 107-115. - PubMed
-
- Beaty RM, Jackson M, Peterson D, et al. Delivery of glucose-6-phosphatase in a canine model for glycogen storage disease, type Ia, with adeno-associated virus (AAV) vectors. Gene Ther. 2002; 9: 1015-1022. - PubMed
-
- Lebherz C, Gao G, Louboutin JP, Millar J, Rader D, Wilson JM. Gene therapy with novel adeno-associated virus vectors substantially diminishes atherosclerosis in a murine model of familial hypercholesterolemia. J Gene Med. 2004;6: 663-672. - PubMed
-
- Ding Z, Georgiev P, Thony B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 2006;13: 587-593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

