Correction of the disease phenotype in canine leukocyte adhesion deficiency using ex vivo hematopoietic stem cell gene therapy
- PMID: 16868255
- PMCID: PMC1895427
- DOI: 10.1182/blood-2006-03-006908
Correction of the disease phenotype in canine leukocyte adhesion deficiency using ex vivo hematopoietic stem cell gene therapy
Abstract
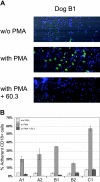
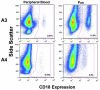
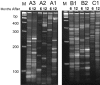
Canine leukocyte adhesion deficiency (CLAD) represents the canine counter-part of the human disease leukocyte adhesion deficiency (LAD). Defects in the leukocyte integrin CD18 adhesion molecule in both CLAD and LAD lead to recurrent, life-threatening bacterial infections. We evaluated ex vivo retroviral-mediated gene therapy in CLAD using 2 nonmyeloablative conditioning regimens--200 cGy total body irradiation (TBI) or 10 mg/kg busulfan--with or without posttransplantation immunosuppression. In 6 of 11 treated CLAD dogs, therapeutic levels of CD18(+) leukocytes were achieved. Conditioning with either TBI or busulfan allowed long-term engraftment, and immunosuppression was not required for efficacy. The percentage of CD18(+) leukocytes in the peripheral blood progressively increased over 6 to 8 months after infusion to levels ranging from 1.26% to 8.37% at 1-year follow-up in the 6 dogs. These levels resulted in reversal or moderation of the severe CLAD phenotype. Linear amplification-mediated polymerase chain reaction assays indicated polyclonality of insertion sites. These results describe ex vivo hematopoietic stem cell gene transfer in a disease-specific, large animal model using 2 clinically applicable conditioning regimens, and they provide support for the use of nonmyeloablative conditioning regimens in preclinical protocols of retroviral-mediated gene transfer for nonmalignant hematopoietic diseases such as LAD.
Figures




References
-
- Anderson DC, Schmalsteig FC, Finegold MJ, et al. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985;152: 668-689. - PubMed
-
- Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogenous mutations in the β subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987;50: 193-202. - PubMed
-
- Thomas C, Le Deist F, Cavazzana-Calvo M, et al. Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood. 1995;86: 1629-1635. - PubMed
-
- Bauer TR Jr, Gu YC, Tuschong LM, et al. Nonmyeloablative hematopoietic stem cell transplantation corrects the disease phenotype in the canine model of leukocyte adhesion deficiency. Exp Hematol. 2005;33: 706-712. - PubMed
-
- Creevy KE, Bauer Jr TR, Tuschong LM, et al. Mixed chimeric hematopoietic stem cell transplant reverses the disease phenotype in canine leukocyte adhesion deficiency. Vet Immunol Immunopathol. 2003;95: 113-121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

