Prevalence of drug-resistance mutations and non-subtype B strains among HIV-infected infants from New York State
- PMID: 16868498
- PMCID: PMC2925654
- DOI: 10.1097/01.qai.0000225871.87456.e7
Prevalence of drug-resistance mutations and non-subtype B strains among HIV-infected infants from New York State
Abstract
Prevalence studies indicate that transmission of drug-resistant HIV has been rising in the adult population, but data from the perinatally infected pediatric population are limited. In this retrospective study, we sequenced the pol region of HIV from perinatally infected infants diagnosed in New York State in 2001-2002. Analyses of drug resistance, subtype diversity, and perinatal antiretroviral exposure were conducted, and the results were compared with those from a previous study of HIV-infected infants identified in 1998-1999. Eight of 42 infants (19.1%) had provirus carrying at least 1 drug-resistance mutation, an increase of 58% over the 1998-1999 results. Mutations conferring resistance to nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, and protease inhibitors were detected in 7.1%, 11.9%, and 2.4% of specimens, respectively. Consistent with previous results, perinatal antiretroviral exposure was not associated with drug resistance (P = 0.70). Phylogenetic analysis indicated that 16.7% of infants were infected with a non-subtype B strain of HIV. It seems that drug-resistant and non-subtype B strains of HIV are becoming increasingly common in the perinatally infected population. Our results highlight the value of resistance testing for all HIV-infected infants upon diagnosis and the need to consider subtype diversity in diagnostic and treatment strategies.
Figures
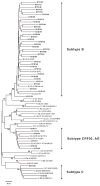
References
-
- Perinatal HIV Guidelines Working Group. Public Health Service Task Force recommendations for the use of antiretroviral drugs in pregnant HIV-1 infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. Feb 25, 2005. Available at: http://aidsinfo.nih.gov/guidelines/perinatal/PER_022405.pdf. - PubMed
-
- Mofenson LM. Successes and challenges in the perinatal HIV-1 epidemic in the United States as illustrated by the HIV-1 serosurvey of childbearing women. Arch Pediatr Adolesc Med. 2004;158:422–425. - PubMed
-
- Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393–1401. - PubMed
-
- Boden D, Hurley A, Zhang L, et al. HIV-1 drug resistance in newly infected individuals. JAMA. 1999;282:1135–1141. - PubMed
-
- Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

