Phellinus linteus sensitises apoptosis induced by doxorubicin in prostate cancer
- PMID: 16868541
- PMCID: PMC2360641
- DOI: 10.1038/sj.bjc.6603277
Phellinus linteus sensitises apoptosis induced by doxorubicin in prostate cancer
Abstract
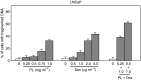
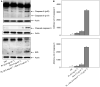
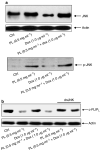
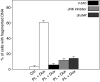
It has been demonstrated that the Phellinus linteus (PL) mushroom, which mainly consists of polysaccharides, possesses antitumour activity. The mechanisms of PL against malignant growth remain unknown. The anticancer drug doxorubicin (Dox) has been shown to induce apoptosis via initiating a caspase cascade. In this investigation, we tested the effect of PL on Dox-induced apoptosis in prostate cancer LNCaP cells. We showed that PL or Dox, at relatively low doses, does not induce apoptosis in the cells. However, combination treatment with low doses of PL and Dox results in a synergistic effect on the induction of apoptosis. In this apoptotic process, caspases 8, 3 and BID are cleaved, and the addition of caspase inhibitor z-VADfmk completely blocks apoptosis. In addition, JNK is activated in response to PL or the combination treatment in LNCaP cells. The suppression of JNK partially inhibits the induction of apoptosis elicited by the co-treatment. These findings indicate that PL has a synergistic effect with Dox to activate caspases in prostate cancer LNCaP cells. Our study also suggests that PL has therapeutic potential to augment the magnitude of apoptosis induced by antiprostate cancer drugs.
Figures

Similar articles
-
Phellinus linteus activates different pathways to induce apoptosis in prostate cancer cells.Br J Cancer. 2007 Feb 26;96(4):583-90. doi: 10.1038/sj.bjc.6603595. Epub 2007 Jan 30. Br J Cancer. 2007. PMID: 17262078 Free PMC article.
-
Phellinus linteus extract induces autophagy and synergizes with 5-fluorouracil to inhibit breast cancer cell growth.Nutr Cancer. 2015;67(2):275-84. doi: 10.1080/01635581.2015.989374. Epub 2015 Jan 26. Nutr Cancer. 2015. PMID: 25622112
-
Doxorubicin enhances TRAIL-induced apoptosis in prostate cancer.Int J Oncol. 2002 May;20(5):949-54. Int J Oncol. 2002. PMID: 11956588
-
Synergistic anticancer activity of doxorubicin and piperlongumine on DU-145 prostate cancer cells - The involvement of carbonyl reductase 1 inhibition.Chem Biol Interact. 2019 Feb 25;300:40-48. doi: 10.1016/j.cbi.2019.01.003. Epub 2019 Jan 3. Chem Biol Interact. 2019. PMID: 30611789
-
A medicinal mushroom: Phellinus linteus.Curr Med Chem. 2008;15(13):1330-5. doi: 10.2174/092986708784534929. Curr Med Chem. 2008. PMID: 18537612 Review.
Cited by
-
Phellinus linteus extract sensitizes advanced prostate cancer cells to apoptosis in athymic nude mice.PLoS One. 2010 Mar 31;5(3):e9885. doi: 10.1371/journal.pone.0009885. PLoS One. 2010. PMID: 20360989 Free PMC article.
-
Phellinus linteus activates different pathways to induce apoptosis in prostate cancer cells.Br J Cancer. 2007 Feb 26;96(4):583-90. doi: 10.1038/sj.bjc.6603595. Epub 2007 Jan 30. Br J Cancer. 2007. PMID: 17262078 Free PMC article.
-
A lethal synergy induced by phellinus linteus and camptothecin11 in colon cancer cells.Oncotarget. 2018 Jan 4;9(5):6308-6319. doi: 10.18632/oncotarget.23918. eCollection 2018 Jan 19. Oncotarget. 2018. PMID: 29464074 Free PMC article.
-
Species identity of Phellinus linteus (sanghuang) extensively used as a medicinal mushroom in Korea.J Microbiol. 2016 Apr;54(4):290-5. doi: 10.1007/s12275-016-5520-2. Epub 2016 Apr 1. J Microbiol. 2016. PMID: 27033204
-
Medicinal mushroom Phellinus linteus as an alternative cancer therapy.Exp Ther Med. 2010 May;1(3):407-411. doi: 10.3892/etm_00000063. Epub 2010 May 1. Exp Ther Med. 2010. PMID: 22993555 Free PMC article.
References
-
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thomberry NA, Wong WW, Yuan J (1996) Human ICE/CED-3 protease nomenclature. Cell 87: 171–179 - PubMed
-
- Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin EM (1999) Minireview: mushrooms, tumors and immunity. Soc Exp Biol Med 221: 281–293 - PubMed
-
- Chang L, Kamata H, Solinas G, Luo J, Maeda S, Venuprasad K, Liu Y, Karin M (2006) The E3 ubiquiting ligase Itch couples JNK activation to TNFα-induced cell death by inducing c-FLIPL Turnover. Cell 124: 601–613 - PubMed
-
- Chihara G, Maeda Y, Hamuro J, Sasaki T, Fumiko F (1969) Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes. Nature 222: 687–688 - PubMed
-
- Chung KS, Choi EC, Kim BK, Kim YS, Park YK (1982) The constituents and culture of Korean Basidiomycetes: antitumor polysaccharides from the cultured mycelia of some Basidiomycetes. Arch Pharm Res 5: 17–20
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

