Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells
- PMID: 16868543
- PMCID: PMC2360674
- DOI: 10.1038/sj.bjc.6603273
Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells
Abstract
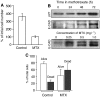
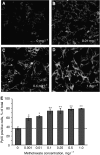
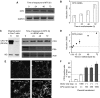
Photodynamic therapy (PDT) using 5-aminolaevulinic acid (ALA) to drive production of an intracellular photosensitiser, protoporphyrin IX (PpIX), is a promising cancer treatment. However, ALA-PDT is still suboptimal for thick or refractory tumours. Searching for new approaches, we tested a known inducer of cellular differentiation, methotrexate (MTX), in combination with ALA-PDT in LNCaP cells. Methotrexate alone promoted growth arrest, differentiation, and apoptosis. Methotrexate pretreatment (1 mg l(-1), 72 h) followed by ALA (0.3 mM, 4 h) resulted in a three-fold increase in intracellular PpIX, by biochemical and confocal analyses. After exposure to 512 nm light, killing was significantly enhanced in MTX-preconditioned cells. The reverse order of treatments, ALA-PDT followed by MTX, yielded no enhancement. Methotrexate caused a similar relative increase in PpIX, whether cells were incubated with ALA, methyl-ALA, or hexyl-ALA, arguing against a major effect upon ALA transport. Searching for an effect among porphyrin synthetic enzymes, we found that coproporphyrinogen oxidase (CPO) was increased three-fold by MTX at the mRNA and protein levels. Transfection of LNCaP cells with a CPO-expressing vector stimulated the accumulation of PpIX. Our data suggest that MTX, when used to modulate intracellular production of endogenous PpIX, may provide a new combination PDT approach for certain cancers.
Figures




Similar articles
-
Methotrexate enhances 5-aminolevulinic acid-mediated photodynamic therapy-induced killing of human SCC4 cells by upregulation of coproporphyrinogen oxidase.J Formos Med Assoc. 2014 Feb;113(2):88-93. doi: 10.1016/j.jfma.2013.12.005. Epub 2014 Jan 30. J Formos Med Assoc. 2014. PMID: 24485831
-
Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo.Clin Cancer Res. 2009 May 15;15(10):3333-43. doi: 10.1158/1078-0432.CCR-08-3054. Epub 2009 May 15. Clin Cancer Res. 2009. PMID: 19447864 Free PMC article.
-
Topical methotrexate pretreatment enhances the therapeutic effect of topical 5-aminolevulinic acid-mediated photodynamic therapy on hamster buccal pouch precancers.J Formos Med Assoc. 2014 Sep;113(9):591-9. doi: 10.1016/j.jfma.2014.03.002. Epub 2014 May 5. J Formos Med Assoc. 2014. PMID: 24811932
-
Pretreatment to enhance protoporphyrin IX accumulation in photodynamic therapy.Dermatology. 2009;218(3):193-202. doi: 10.1159/000183753. Epub 2008 Dec 11. Dermatology. 2009. PMID: 19077380 Review.
-
The use of 5-aminolaevulinic acid as a photosensitiser in photodynamic therapy and photodiagnosis.Photochem Photobiol Sci. 2002 Mar;1(3):158-68. doi: 10.1039/b201027p. Photochem Photobiol Sci. 2002. PMID: 12659511 Review.
Cited by
-
Chemophototherapy: An Emerging Treatment Option for Solid Tumors.Adv Sci (Weinh). 2016 May 24;4(1):1600106. doi: 10.1002/advs.201600106. eCollection 2017 Jan. Adv Sci (Weinh). 2016. PMID: 28105389 Free PMC article. Review.
-
Her2 oncogene transformation enhances 5-aminolevulinic acid-mediated protoporphyrin IX production and photodynamic therapy response.Oncotarget. 2016 Sep 6;7(36):57798-57810. doi: 10.18632/oncotarget.11058. Oncotarget. 2016. PMID: 27527860 Free PMC article.
-
Development of laser ionization techniques for evaluation of the effect of cancer drugs using imaging mass spectrometry.Int J Mol Sci. 2014 Jun 25;15(7):11234-44. doi: 10.3390/ijms150711234. Int J Mol Sci. 2014. PMID: 24968266 Free PMC article.
-
Photodynamic therapy for the precise treatment of localized prostate cancer.Front Oncol. 2025 Feb 5;15:1454392. doi: 10.3389/fonc.2025.1454392. eCollection 2025. Front Oncol. 2025. PMID: 39975598 Free PMC article. Review.
-
Mechanism of differentiation-enhanced photodynamic therapy for cancer: upregulation of coproporphyrinogen oxidase by C/EBP transcription factors.Mol Cancer Ther. 2013 Aug;12(8):1638-50. doi: 10.1158/1535-7163.MCT-13-0047. Epub 2013 May 16. Mol Cancer Ther. 2013. PMID: 23686770 Free PMC article.
References
-
- Berkowitz RS, Goldstein DP, Bernstein MR (1986) Ten year's experience with methotrexate and folinic acid as primary therapy for gestational trophoblastic disease. Gynecol Oncol 23: 111–118 - PubMed
-
- Bickers DR, Pathak MA, Lim HW (1999) The Porphyrias. In Dermatology in General Medicine Freedberg IMEA (ed) Vol. 2, pp 1766–1803. New York: McGraw-Hill
-
- Bodner AJ, Ting RC, Gallo RC (1981) Induction of differentiation of human promyelocytic leukemia cells (HL-60) by nucleosides and methotrexate. J Natl Cancer Inst 67: 1025–1030 - PubMed
-
- Burres NS, Cass CE (1987) Inhibition of methotrexate-induced differentiation of cultured human choriocarcinoma (BeWo) cells by thymidine. Cancer Res 47: 5059–5064 - PubMed
-
- Campbell MJ, Elstner E, Holden S, Uskokovic M, Koeffler HP (1997) Inhibition of proliferation of prostate cancer cells by a 19-nor-hexafluoride vitamin D3 analogue involves the induction of p21waf1, p27kip1 and E-cadherin. J Mol Endocrinol 19: 15–27 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

