Guided selection of an anti-gamma-seminoprotein human Fab for antibody directed enzyme prodrug therapy of prostate cancer
- PMID: 16868778
- PMCID: PMC11030898
- DOI: 10.1007/s00262-006-0202-2
Guided selection of an anti-gamma-seminoprotein human Fab for antibody directed enzyme prodrug therapy of prostate cancer
Abstract
Background: The HAMA response is a major challenge when murine antibodies are repeatedly administered for antibody directed enzyme prodrug therapy in vivo. In this study we have achieved humanization of the anti-gamma-seminoprotein E(4)B(7) murine mAb by guided selection.
Methods: Using optimal Ig Fab primers, human Fd and CL gene repertoires were amplified by RT-PCR from PBMCs of prostate cancer patients. The human Lc gene repertoire was first paired with the murine Fd gene of E(4)B(7) mAb to construct a pComb3X hybrid Fab display library. This hybrid library was screened with purified gamma-seminoprotein antigen. The human Fd gene repertoire was then paired with the selected human Lc to construct a fully human Fab library. After four more rounds of panning, completely human Fab antibodies specific for gamma-seminoprotein were selected and further identified.
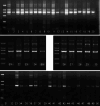
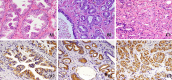
Results: First, using the E(4)B(7) Fd gene as a template, light chain shuffling was achieved by panning the hybrid library. Then, using the selected Lc as a template, a human Fab antibody against gamma-seminoprotein was produced through heavy chain Fd shuffling. Western blotting, ELISA, and flow cytometry results demonstrated that the resulting human Fab antibody resembled the parental E(4)B(7) mAb in that they both recognized the same epitope with similar affinities. Fluorescent cell staining and immunohistochemistry analysis further confirmed that this newly constructed human anti-gamma-seminoprotein Fab antibody indeed specifically bound prostate cancer cells and tissue.
Conclusions: Through guided-selection, we successfully produced a human anti-gamma-seminoprotein Fab antibody. This work lays the foundation for optimal antibody-directed enzyme prodrug therapy of prostate cancer using a fully human Fab antibody.
Figures






Similar articles
-
[Screening of human anti-gamma-seminoprotein light chain guided with the murine Fd fragment].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006 Mar;22(2):196-9. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2006. PMID: 16507258 Chinese.
-
Isolating human antibody against human hepatocellular carcinoma by guided-selection.Cancer Biol Ther. 2005 Dec;4(12):1374-80. doi: 10.4161/cbt.4.12.2273. Epub 2005 Dec 25. Cancer Biol Ther. 2005. PMID: 16319526
-
[Preparation of human Fab antibodies against HAb18G by guided selection and chain shuffling technique].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2004 Jul;20(4):437-40. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2004. PMID: 15207089 Chinese.
-
[Prokaryotic expression and bioactivity identification of Fab against human gamma-seminoprotein].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003 Sep;19(5):483-5. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003. PMID: 15169664 Chinese.
-
Humanization of a mouse monoclonal antibody neutralizing TNF-alpha by guided selection.J Immunol Methods. 2000 Jul 31;241(1-2):171-84. doi: 10.1016/s0022-1759(00)00203-9. J Immunol Methods. 2000. PMID: 10915859
Cited by
-
Effects of different interchain linkers on biological activity of an anti-prostate cancer single-chain bispecific antibody.Theor Biol Med Model. 2015 Aug 6;12:14. doi: 10.1186/s12976-015-0010-5. Theor Biol Med Model. 2015. PMID: 26246000 Free PMC article.
-
Isolation and molecular characterization of novel glucarpidases: Enzymes to improve the antibody directed enzyme pro-drug therapy for cancer treatment.PLoS One. 2018 Apr 26;13(4):e0196254. doi: 10.1371/journal.pone.0196254. eCollection 2018. PLoS One. 2018. PMID: 29698433 Free PMC article.
-
Phage display library selection of a hypoxia-binding scFv antibody for liver cancer metabolic marker discovery.Oncotarget. 2016 Jun 21;7(25):38105-38121. doi: 10.18632/oncotarget.9460. Oncotarget. 2016. PMID: 27203546 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

