Codeine and 6-acetylcodeine analgesia in mice
- PMID: 16868817
- PMCID: PMC11520751
- DOI: 10.1007/s10571-006-9101-5
Codeine and 6-acetylcodeine analgesia in mice
Abstract
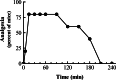
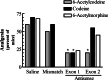
1. Acetylation of morphine at the 6-position changes its pharmacology. To see if similar changes are seen with codeine, we examined the analgesic actions of codeine and 6-acetylcodeine. 2. Like codeine, 6-acetylcodeine is an effective analgesic systemically, supraspinally and spinally, with a potency approximately a third that of codeine. 3. The sensitivity of 6-acetylcodeine analgesia to the mu-selective antagonists beta-FNA and naloxonazine confirmed its classification as a mu opioid. However, it differed from the other mu analgesics in other paradigms. 4. Antisense mapping revealed the sensitivity of 6-acetylcodeine to probes targeting exons 1 and 2 of the mu opioid receptor gene (Oprm), a profile distinct from either codeine or morphine. Although heroin analgesia also is sensitive to antisense targeting exons 1 and 2, heroin analgesia also is sensitive to the antagonist 3-O-methylnaltrexone, while 6-acetylcodeine analgesia is not. 5. Thus, 6-acetylcodeine is an effective mu opioid analgesic with a distinct pharmacological profile.
Figures

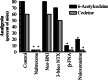

Similar articles
-
Pharmacological characterization of dihydromorphine, 6-acetyldihydromorphine and dihydroheroin analgesia and their differentiation from morphine.Eur J Pharmacol. 2004 May 25;492(2-3):123-30. doi: 10.1016/j.ejphar.2004.03.050. Eur J Pharmacol. 2004. PMID: 15178355
-
Pharmacological characterization of morphine-6-sulfate and codeine-6-sulfate.Brain Res. 1999 Sep 18;842(1):1-5. doi: 10.1016/s0006-8993(99)01766-7. Brain Res. 1999. PMID: 10526089
-
Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors.Brain Res. 2006 Apr 14;1083(1):61-9. doi: 10.1016/j.brainres.2006.01.095. Epub 2006 Mar 10. Brain Res. 2006. PMID: 16530171
-
Codeine analgesia is due to codeine-6-glucuronide, not morphine.Int J Clin Pract. 2000 Jul-Aug;54(6):395-8. Int J Clin Pract. 2000. PMID: 11092114 Review.
-
The pharmacology of mu analgesics: from patients to genes.Neuroscientist. 2001 Jun;7(3):220-31. doi: 10.1177/107385840100700307. Neuroscientist. 2001. PMID: 11499401 Review.
Cited by
-
Endogenous opiates and behavior: 2006.Peptides. 2007 Dec;28(12):2435-513. doi: 10.1016/j.peptides.2007.09.002. Epub 2007 Sep 11. Peptides. 2007. PMID: 17949854 Free PMC article. Review.
References
-
- Bare, L. A., Mansson, E., and Yang, D. (1994). Expression of two variants of the human μ opioid receptor mRNA in SK-N-SH cells and human brain. FEBS Lett.354:213–216. - PubMed
-
- Baron, A., Shuster, L., Elefterhiou, B. E., and Bailey, D. W. (1975). Opiate receptors in mice: Genetic differences. Life Sci.17:633–640. - PubMed
-
- Brown, G. P., Yang, K., King, M. A., Rossi, G. C., Leventhal, L., Chang, A., and Pasternak, G. W. (1997a). 3-Methoxynaltrexone, a selective heroin/morphine-6β-glucuronide antagonist. FEBS Lett.412:35–38. - PubMed
-
- Brown, G. P., Yang, K., Ouerfelli, O., Standifer, K. M., Byrd, D., and Pasternak, G. W. (1997b). 3H-Morphine-6β-glucuronide binding in brain membranes and in an MOR-1 transfected cell line. J. Pharmacol. Exp. Ther.282:1291–1297. - PubMed
-
- Hahn, E. F., and Pasternak, G. W. (1982). Naloxonazine, a potent, long-acting inhibitor of opiate binding sites. Life Sci.31:1385–1388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

