Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance
- PMID: 16869817
- PMCID: PMC2000709
- DOI: 10.1111/j.1365-2125.2006.02725.x
Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance
Abstract
What is already known about this subject: *Effects of size, renal function, age (postnatal age, gestational age and postmenstrual age) as predictors of vancomycin clearance in premature neonates are established, but the relative contribution of each component remains poorly quantified, largely because these variables are closely correlated. *We have quantified the covariates contributing to vancomycin clearance population parameter variability in order to establish the major covariates required for dosing predictions. Size, standardized using allometric models, was the primary covariate used in our analysis.
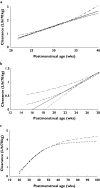
What this study adds: *Size explained 49.8%, postmenstrual age 18.2% and renal function 14.1% of clearance variability. *Descriptors of the relationship between age and clearance in premature neonates vary. *The use of a variable slope sigmoidal model to describe the relationship between clearance and postmenstrual age predicted an adult clearance of 3.79 l h(-1) 70 kg(-1) (95% confidence interval 2.76, 4.98) from premature neonatal data.
Aim: To identify and quantify factors describing the variability of vancomycin clearance in premature neonates.
Methods: Population pharmacokinetics were estimated (NONMEM) in 214 neonates [postmenstrual age (PMA) 30.4 weeks, range 24-34 weeks; postnatal age 11.9 days, range 1-27 days; weight 1.30 kg, range 0.42-2.6 kg] using therapeutic drug monitoring data. Covariate analysis included weight, PMA, serum creatinine, use of inotropes or ibuprofen, positive blood culture and respiratory support. A one-compartment linear disposition model with zero order input and first-order elimination was used to describe the data (604 observations).
Results: The population estimate for volume of distribution (V) was 39 l 70 kg(-1) (coefficient of variation 19.4%). Clearance (CL) increased from 0.897 l h(-1) 70 kg(-1) at 24 weeks PMA to 2.02 l h(-1) 70 kg(-1) by 34 weeks PMA. The between-subject variability for CL was 18.6% and the between-occasion variability was 12.2%. The use of ibuprofen reduced clearance, but this effect was attributable to reduced renal function. Overall, 82% of the variability of CL was predictable. Size explained 49.8%, PMA 18.2% and renal function 14.1%. The use of a variable slope sigmoidal model to describe the relationship between clearance and PMA predicted an adult clearance of 3.79 l h(-1) 70 kg(-1) (95% confidence interval 2.76, 4.98).
Conclusions: Size, renal function and PMA are the major contributors to clearance variability in premature neonates. The small (18%) unexplained variability in clearance suggests target concentration intervention is unnecessary if size, age and renal function are used to predict the dose. Extrapolation to an adult clearance from neonatal data is possible using allometric size models and a function describing clearance maturation.
Figures


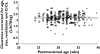

References
-
- de Hoog M, Mouton JW, van den Anker JN. New dosing strategies for antibacterial agents in the neonate. Semin Fetal Neonatal Med. 2005;10:185–94. - PubMed
-
- de Hoog M, Mouton JW, van den Anker JN. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 2004;43:417–40. - PubMed
-
- Frattarelli DA, Ergun H, Lulic-Botica M, Lehr VT, Aranda JV. Vancomycin elimination in human infants with intrauterine growth retardation. Pediatr Infect Dis J. 2005;24:979–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

