A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors
- PMID: 16870701
- PMCID: PMC1635374
- DOI: 10.1091/mbc.e06-05-0421
A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors
Abstract
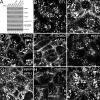
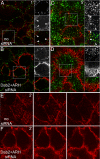
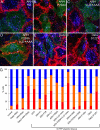
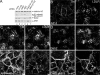
Sorting of transmembrane cargo into clathrin-coated vesicles requires endocytic adaptors, yet RNA interference (RNAi)-mediated gene silencing of the AP-2 adaptor complex only disrupts internalization of a subset of clathrin-dependent cargo. This suggests alternate clathrin-associated sorting proteins participate in cargo capture at the cell surface, and a provocative recent proposal is that discrete endocytic cargo are sorted into compositionally and functionally distinct clathrin coats. We show here that the FXNPXY-type internalization signal within cytosolic domain of the LDL receptor is recognized redundantly by two phosphotyrosine-binding domain proteins, Dab2 and ARH; diminishing both proteins by RNAi leads to conspicuous LDL receptor accumulation at the cell surface. AP-2-dependent uptake of transferrin ensues relatively normally in the absence of Dab2 and ARH, clearly revealing delegation of sorting operations at the bud site. AP-2, Dab2, ARH, transferrin, and LDL receptors are all present within the vast majority of clathrin structures at the surface, challenging the general existence of specialized clathrin coats for segregated internalization of constitutively internalized cargo. However, Dab2 expression is exceptionally low in hepatocytes, likely accounting for the pathological hypercholesterolemia that accompanies ARH loss.
Figures



Similar articles
-
The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH.J Cell Sci. 2006 Oct 15;119(Pt 20):4235-46. doi: 10.1242/jcs.03217. Epub 2006 Sep 19. J Cell Sci. 2006. PMID: 16984970
-
The clathrin adaptor Dab2 recruits EH domain scaffold proteins to regulate integrin β1 endocytosis.Mol Biol Cell. 2012 Aug;23(15):2905-16. doi: 10.1091/mbc.E11-12-1007. Epub 2012 May 30. Mol Biol Cell. 2012. PMID: 22648170 Free PMC article.
-
The clathrin adaptor proteins ARH, Dab2, and numb play distinct roles in Niemann-Pick C1-Like 1 versus low density lipoprotein receptor-mediated cholesterol uptake.J Biol Chem. 2014 Nov 28;289(48):33689-700. doi: 10.1074/jbc.M114.593764. Epub 2014 Oct 20. J Biol Chem. 2014. PMID: 25331956 Free PMC article.
-
Cargo recognition during clathrin-mediated endocytosis: a team effort.Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
-
Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection.J Cell Biol. 2003 Oct 27;163(2):203-8. doi: 10.1083/jcb.200309175. J Cell Biol. 2003. PMID: 14581447 Free PMC article. Review.
Cited by
-
S-nitrosylation of ARH is required for LDL uptake by the LDL receptor.J Lipid Res. 2013 Jun;54(6):1550-1559. doi: 10.1194/jlr.M033167. Epub 2013 Apr 7. J Lipid Res. 2013. PMID: 23564733 Free PMC article.
-
Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides.J Cell Mol Med. 2007 Jul-Aug;11(4):670-84. doi: 10.1111/j.1582-4934.2007.00062.x. J Cell Mol Med. 2007. PMID: 17760832 Free PMC article. Review.
-
Dab2 inhibits the cholesterol-dependent activation of JNK by TGF-β.Mol Biol Cell. 2014 May;25(10):1620-8. doi: 10.1091/mbc.E13-09-0537. Epub 2014 Mar 19. Mol Biol Cell. 2014. PMID: 24648493 Free PMC article.
-
Negative regulation of the endocytic adaptor disabled-2 (Dab2) in mitosis.J Biol Chem. 2011 Feb 18;286(7):5392-403. doi: 10.1074/jbc.M110.161851. Epub 2010 Nov 19. J Biol Chem. 2011. PMID: 21097498 Free PMC article.
-
Double-membrane gap junction internalization requires the clathrin-mediated endocytic machinery.FEBS Lett. 2008 Aug 20;582(19):2887-92. doi: 10.1016/j.febslet.2008.07.024. Epub 2008 Jul 24. FEBS Lett. 2008. PMID: 18656476 Free PMC article.
References
-
- Anderson R. G., Vasile E., Mello R. J., Brown M. S. Immunocytochemical visualization of coated pits and vesicles in human fibroblasts: relation to low density lipoprotein receptor distribution. Cell. 1978;15:919–933. - PubMed
-
- Arca M. Autosomal recessive hypercholesterolaemia in Sardinia, Italy, and mutations in ARH: a clinical and molecular genetic analysis. Lancet. 2002;359:841–847. - PubMed
-
- Barriere H., Nemes C., Lechardeur D., Khan-Mohammad M., Fruh K. Molecular basis of Ub-dependent internalization of membrane proteins in mammalian cells. Traffic. 2006;7:282–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

