Control of fast-reaching movements by muscle synergy combinations
- PMID: 16870725
- PMCID: PMC6674215
- DOI: 10.1523/JNEUROSCI.0830-06.2006
Control of fast-reaching movements by muscle synergy combinations
Abstract
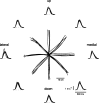
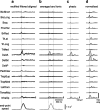
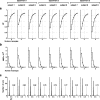
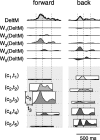
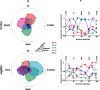
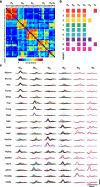
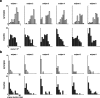
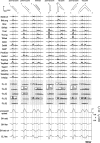
How the CNS selects the appropriate muscle patterns to achieve a behavioral goal is an open question. To gain insight into this process, we characterized the spatiotemporal organization of the muscle patterns for fast-reaching movements. We recorded electromyographic activity from up to 19 shoulder and arm muscles during point-to-point movements between a central location and 8 peripheral targets in each of 2 vertical planes. We used an optimization algorithm to identify a set of time-varying muscle synergies, i.e., the coordinated activations of groups of muscles with specific time-varying profiles. For each one of nine subjects, we extracted four or five synergies whose combinations, after scaling in amplitude and shifting in time each synergy independently for each movement condition, explained 73-82% of the data variation. We then tested whether these synergies could reconstruct the muscle patterns for point-to-point movements with different loads or forearm postures and for reversal and via-point movements. We found that reconstruction accuracy remained high, indicating generalization across these conditions. Finally, the synergy amplitude coefficients were directionally tuned according to a cosine function with a preferred direction that showed a smaller variability with changes of load, posture, and endpoint than the preferred direction of individual muscles. Thus the complex spatiotemporal characteristics of the muscles patterns for reaching were captured by the combinations of a small number of components, suggesting that the mechanisms involved in the generation of the muscle patterns exploit this low dimensionality to simplify control.
Figures







References
-
- Bartlett MS (1950). Tests of significance in factor analysis. Br J Psychol 3:77–85.
-
- Basilevsky A (1994). In: Statistical factor analysis and related methods: theory and applications New York: Wiley.
-
- Bastian AJ, Martin TA, Keating JG, Thach WT (1996). Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 76:492–509. - PubMed
-
- Batschelet E (1981). In: Circular statistics in biology London: Academic.
-
- Bhushan N, Shadmehr R (1999). Computational nature of human adaptive control during learning of reaching movements in force fields. Biol Cybern 81:39–60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
