Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice
- PMID: 16870730
- PMCID: PMC6674222
- DOI: 10.1523/JNEUROSCI.1526-06.2006
Tenascin-R restricts posttraumatic remodeling of motoneuron innervation and functional recovery after spinal cord injury in adult mice
Abstract
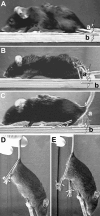
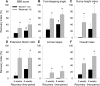
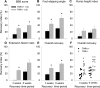
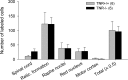
Tenascin-R (TNR) is an extracellular glycoprotein in the CNS implicated in neural development and plasticity. Its repellent properties for growing axons in a choice situation with a conducive substrate in vitro have indicated that TNR may impede regeneration in the adult mammalian CNS. Here we tested whether constitutive lack of TNR has beneficial impacts on recovery from spinal cord injury in adult mice. Using the Basso, Beattie, Bresnahan (BBB) locomotor rating scale, we found that open-field locomotion in TNR-deficient (TNR-/-) mice recovered better that in wild-type (TNR+/+) littermates after compression of the thoracic spinal cord. We also designed, validated, and applied a motion analysis approach allowing numerical assessment of motor functions. We found, in agreement with the BBB score, that functions requiring low levels of supraspinal control such as plantar stepping improved more in TNR-/- mice. This was not the case for motor tasks demanding precision such as ladder climbing. Morphological analyses revealed no evidence that improved recovery of some functions in the mutant mice were attributable to enhanced tissue sparing or axonal regrowth. Estimates of perisomatic puncta revealed reduced innervation by cholinergic and GABAergic terminals around motoneurons in intact TNR-/- compared with TNR+/+ mice. Relative to nonlesioned animals, spinal cord repair was associated with increase in GABAergic and decrease of glutamatergic puncta in TNR-/- but not in TNR+/+ mice. Our results suggest that TNR restricts functional recovery by limiting posttraumatic remodeling of synapses around motoneuronal cell bodies where TNR is normally expressed in perineuronal nets.
Figures



References
-
- Basso DM (2004). Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma 21:395–404. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W (1996). MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma 13:343–359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
