Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain
- PMID: 16870743
- PMCID: PMC6674206
- DOI: 10.1523/JNEUROSCI.2211-06.2006
Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain
Abstract
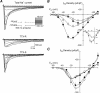
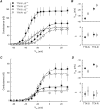
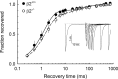
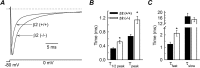
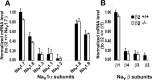
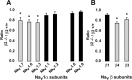
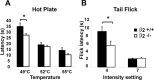
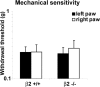
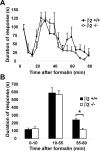
Voltage-gated sodium channel (Na(v)1) beta2 subunits modulate channel gating, assembly, and cell-surface expression in CNS neurons in vitro and in vivo. beta2 expression increases in sensory neurons after nerve injury, and development of mechanical allodynia in the spared nerve injury model is attenuated in beta2-null mice. Thus, we hypothesized that beta2 modulates electrical excitability in dorsal root ganglion (DRG) neurons in vivo. We compared sodium currents (I(Na)) in small DRG neurons from beta2+/+ and beta2-/- mice to determine the effects of beta2 on tetrodotoxin-sensitive (TTX-S) and tetrodotoxin-resistant (TTX-R) Na(v)1 in vivo. Small-fast DRG neurons acutely isolated from beta2-/- mice showed significant decreases in TTX-S I(Na) compared with beta2+/+ neurons. This decrease included a 51% reduction in maximal sodium conductance with no detectable changes in the voltage dependence of activation or inactivation. TTX-S, but not TTX-R, I(Na) activation and inactivation kinetics in these cells were slower in beta2(-/-) mice compared with controls. The selective regulation of TTX-S I(Na) was supported by reductions in transcript and protein levels of TTX-S Na(v)1s, particularly Na(v)1.7. Low-threshold mechanical sensitivity was preserved in beta2-/- mice, but they were more sensitive to noxious thermal stimuli than wild type whereas their response during the late phase of the formalin test was attenuated. Our results suggest that beta2 modulates TTX-S Na(v)1 mRNA and protein expression resulting in increased TTX-S I(Na) and increases the rates of TTX-S Na(v)1 activation and inactivation in small-fast DRG neurons in vivo. TTX-R I(Na) were not significantly modulated by beta2.
Figures


References
-
- Abdulla FA, Smith PA (2001). Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol 85:644–658. - PubMed
-
- Abdulla FA, Smith PA (2002). Changes in Na(+) channel currents of rat dorsal root ganglion neurons following axotomy and axotomy-induced autotomy. J Neurophysiol 88:2518–2529. - PubMed
-
- Akopian AN, Sivilotti L, Wood JN (1996). A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379:257–262. - PubMed
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN (1999). The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2:541–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
