Encoding and decoding touch location in the leech CNS
- PMID: 16870746
- PMCID: PMC6674225
- DOI: 10.1523/JNEUROSCI.5472-05.2006
Encoding and decoding touch location in the leech CNS
Abstract
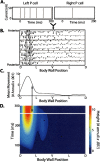
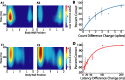
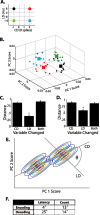
Spike times encode stimulus values in many sensory systems, but it is generally unknown whether such temporal variations are decoded (i.e., whether they influence downstream networks that control behavior). In the present study, we directly address this decoding problem by quantifying both sensory encoding and decoding in the leech. By mechanically stimulating the leech body wall while recording from mechanoreceptors, we show that pairs of leech sensory neurons with overlapping receptive fields encode touch location by their relative latencies, number of spikes, and instantaneous firing rates, with relative latency being the most accurate indicator of touch location. We then show that the relative latency and count are decoded by manipulating these variables in sensory neuron pairs while simultaneously monitoring the resulting behavior. Although both variables are important determinants of leech behavior, the decoding mechanisms are more sensitive to changes in relative spike count than changes in relative latency.
Figures


Similar articles
-
Multiplexed Population Coding of Stimulus Properties by Leech Mechanosensory Cells.J Neurosci. 2016 Mar 30;36(13):3636-47. doi: 10.1523/JNEUROSCI.1753-15.2016. J Neurosci. 2016. PMID: 27030751 Free PMC article.
-
Representation of touch location by a population of leech sensory neurons.J Neurophysiol. 1998 Nov;80(5):2584-92. doi: 10.1152/jn.1998.80.5.2584. J Neurophysiol. 1998. PMID: 9819265
-
Frequency coding of positional information by an identified neuron, the AP cell, in the leech, Whitmania pigra.Brain Res Bull. 2001 Dec;56(6):511-5. doi: 10.1016/s0361-9230(01)00609-8. Brain Res Bull. 2001. PMID: 11786234
-
Transduction and encoding sensory information by skin mechanoreceptors.Pflugers Arch. 2015 Jan;467(1):109-19. doi: 10.1007/s00424-014-1651-7. Epub 2014 Nov 23. Pflugers Arch. 2015. PMID: 25416542 Review.
-
Texture coding in the whisker system.Curr Opin Neurobiol. 2010 Jun;20(3):313-8. doi: 10.1016/j.conb.2010.02.014. Epub 2010 Mar 17. Curr Opin Neurobiol. 2010. PMID: 20299205 Review.
Cited by
-
Widespread inhibition proportional to excitation controls the gain of a leech behavioral circuit.Neuron. 2008 Jan 24;57(2):276-289. doi: 10.1016/j.neuron.2007.11.028. Neuron. 2008. PMID: 18215624 Free PMC article.
-
Intrinsic frequency response patterns in mechano-sensory neurons of the leech.Biol Open. 2017 Jul 15;6(7):993-999. doi: 10.1242/bio.023960. Biol Open. 2017. PMID: 28546342 Free PMC article.
-
Caterpillar crawling over irregular terrain: anticipation and local sensing.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010 Jun;196(6):397-406. doi: 10.1007/s00359-010-0525-5. Epub 2010 Apr 23. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010. PMID: 20414659
-
Non-synaptic Plasticity in Leech Touch Cells.Front Physiol. 2019 Nov 27;10:1444. doi: 10.3389/fphys.2019.01444. eCollection 2019. Front Physiol. 2019. PMID: 31827443 Free PMC article.
-
Synaptic input and temperature influence sensory coding in a mechanoreceptor.Front Cell Neurosci. 2023 Sep 12;17:1233730. doi: 10.3389/fncel.2023.1233730. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37771930 Free PMC article.
References
-
- Amassian VE (1953). Evoked single cortical unit activity in the somatic sensory areas. Electroencephalogr Clin Neurophysiol Suppl 5:415–438. - PubMed
-
- Baca SM, Thomson EE, Kristan WB (2005). Location and intensity discrimination in the leech local bend response quantified using optic flow and principal components analysis. J Neurophysiol 93:3560–3572. - PubMed
-
- Cholewiak RW (1999). The perception of tactile distance: influences of body site, space, and time. Perception 28:851–875. - PubMed
-
- Duda RO, Hart PE, Stork DG (2000). In: Pattern classification New York: Wiley-Interscience.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
