Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge
- PMID: 16870749
- PMCID: PMC1538671
- DOI: 10.1128/AAC.00181-06
Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge
Abstract
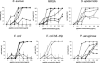
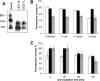
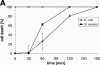
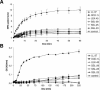
Dermcidin (DCD) is a recently described antimicrobial peptide, which is constitutively expressed in eccrine sweat glands and transported via sweat to the epidermal surface. By postsecretory proteolytic processing in sweat the dermcidin protein gives rise to several truncated DCD peptides which differ in length and net charge. In order to understand the mechanism of antimicrobial activity, we analyzed the spectrum of activity of several naturally processed dermcidin-derived peptides, the secondary structure in different solvents, and the ability of these peptides to interact with or permeabilize the bacterial membrane. Interestingly, although all naturally processed DCD peptides can adopt an alpha-helical conformation in solvents, they have a diverse and partially overlapping spectrum of activity against gram-positive and gram-negative bacteria. This indicates that the net charge and the secondary structure of the peptides are not important for the toxic activity. Furthermore, using carboxyfluorescein-loaded liposomes, membrane permeability studies and electron microscopy we investigated whether DCD peptides are able to permeabilize bacterial membranes. The data convincingly show that irrespective of charge the different DCD peptides are not able to permeabilize bacterial membranes. However, bacterial mutants lacking specific cell envelope modifications exhibited different susceptibilities to killing by DCD peptides than wild-type bacterial strains. Finally, immunoelectron microscopy studies indicated that DCD peptides are able to bind to the bacterial surface; however, signs of membrane perturbation were not observed. These studies indicate that DCD peptides do not exert their activity by permeabilizing bacterial membranes.
Figures



References
-
- Baechle, D., T. Flad, A. Cansier, H. Steffen, B. Schittek, J. Tolson, T. Herrmann, H. Dihazi, A. Beck, G. A. Mueller, M. Mueller, S. Stevanovic, C. Garbe, C. A. Mueller, and H. Kalbacher. 2006. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of DCD-1L. J. Biol. Chem. 281:5406-5415. - PubMed
-
- Beisswenger, C., and R. Bals. 2005. Functions of antimicrobial peptides in host defense and immunity. Curr. Protein Peptide Sci. 6:255-264. - PubMed
-
- Blondelle, S. E., K. Lohner, and M. Aguilar. 1999. Lipid-induced conformation and lipid-binding properties of cytolytic and antimicrobial peptides: determination and biological specificity. Biochim. Biophys. Acta 1462:89-108. - PubMed
-
- Breukink, E., C. van Kraaij, R. A. Demel, R. J. Siezen, O. P. Kuipers, and B. de Kruijff. 1997. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry 36:6968-6976. - PubMed
-
- Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

