Physicochemical properties that enhance discriminative antibacterial activity of short dermaseptin derivatives
- PMID: 16870756
- PMCID: PMC1538664
- DOI: 10.1128/AAC.00030-06
Physicochemical properties that enhance discriminative antibacterial activity of short dermaseptin derivatives
Abstract
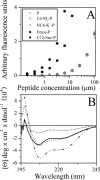
Antimicrobial peptides are widely believed to exert their effects by nonspecific mechanisms. We assessed the extent to which physicochemical properties can be exploited to promote discriminative activity by manipulating the N-terminal sequence of the 13-mer dermaseptin derivative K(4)-S4(1-13) (P). Inhibitory activity determined in culture media against 16 strains of bacteria showed that when its hydrophobicity and charge were changed, P became predominantly active against either gram-positive or gram-negative bacteria. Thus, conjugation of various aminoacyl-lysin moieties (e.g., aminohexyl-K-P) led to inactivity against gram-positive bacteria (MIC(50) > 50 microM) but potent activity against gram-negative bacteria (MIC(50), 6.2 microM). Conversely, conjugation of equivalent acyls to the substituted analog M(4)-S4(1-13) (e.g., hexyl-M(4)-P) led to inactivity against gram-negative bacteria (MIC(50) > 50 microM) but potent activity against gram-positive bacteria (MIC(50), 3.1 microM). Surface plasmon resonance experiments, used to investigate peptides' binding properties to lipopolysaccharide-containing idealized phospholipid membranes, suggest that although the acylated derivatives have increased lipophilic properties with parallel antibacterial behavior, hydrophobic derivatives are prevented from reaching the cytoplasmic membranes of gram-negative bacteria. Moreover, unlike modifications that enhanced the activity against gram-positive bacteria, which also enhanced hemolysis, we found that modifications that enhanced activity against gram-negative bacteria generally reduced hemolysis. Thus, compared with the clinically tested peptides MSI-78 and IB-367, the dermaseptin derivative aminohexyl-K-P performed similarly in terms of potency and bactericidal kinetics but was significantly more selective in terms of discrimination between bacteria and human erythrocytes. Overall, the data suggest that similar strategies maybe useful to derive potent and safe compounds from known antimicrobial peptides.
Figures



Similar articles
-
Acyl-substituted dermaseptin S4 derivatives with improved bactericidal properties, including on oral microflora.Antimicrob Agents Chemother. 2006 Dec;50(12):4153-60. doi: 10.1128/AAC.00750-06. Epub 2006 Oct 16. Antimicrob Agents Chemother. 2006. PMID: 17043126 Free PMC article.
-
In vitro discriminative antipseudomonal properties resulting from acyl substitution of N-terminal sequence of dermaseptin s4 derivatives.Chem Biol. 2007 Jan;14(1):75-85. doi: 10.1016/j.chembiol.2006.11.009. Chem Biol. 2007. PMID: 17254954
-
Mechanism of antibacterial action of dermaseptin B2: interplay between helix-hinge-helix structure and membrane curvature strain.Biochemistry. 2009 Jan 20;48(2):313-27. doi: 10.1021/bi802025a. Biochemistry. 2009. PMID: 19113844
-
An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes.Biochim Biophys Acta. 2016 May;1858(5):958-70. doi: 10.1016/j.bbamem.2015.10.011. Epub 2015 Oct 21. Biochim Biophys Acta. 2016. PMID: 26498397 Review.
-
Update of peptides with antibacterial activity.Curr Med Chem. 2012;19(36):6188-98. Curr Med Chem. 2012. PMID: 22978329 Review.
Cited by
-
Bacterial capture by peptide-mimetic oligoacyllysine surfaces.Appl Environ Microbiol. 2010 May;76(10):3301-7. doi: 10.1128/AEM.00532-10. Epub 2010 Apr 2. Appl Environ Microbiol. 2010. PMID: 20363797 Free PMC article.
-
Impact of self-assembly properties on antibacterial activity of short acyl-lysine oligomers.Antimicrob Agents Chemother. 2008 Dec;52(12):4308-14. doi: 10.1128/AAC.00656-08. Epub 2008 Oct 6. Antimicrob Agents Chemother. 2008. PMID: 18838600 Free PMC article.
-
The Spectrum of Design Solutions for Improving the Activity-Selectivity Product of Peptide Antibiotics against Multidrug-Resistant Bacteria and Prostate Cancer PC-3 Cells.Molecules. 2020 Aug 1;25(15):3526. doi: 10.3390/molecules25153526. Molecules. 2020. PMID: 32752241 Free PMC article.
-
Antibiotic Hybrids: the Next Generation of Agents and Adjuvants against Gram-Negative Pathogens?Clin Microbiol Rev. 2018 Mar 14;31(2):e00077-17. doi: 10.1128/CMR.00077-17. Print 2018 Apr. Clin Microbiol Rev. 2018. PMID: 29540434 Free PMC article. Review.
-
A miniature mimic of host defense peptides with systemic antibacterial efficacy.FASEB J. 2010 Jun;24(6):1904-13. doi: 10.1096/fj.09-149427. Epub 2010 Feb 2. FASEB J. 2010. PMID: 20124435 Free PMC article.
References
-
- Allende, D., and T. J. McIntosh. 2003. Lipopolysaccharides in bacterial membranes act like cholesterol in eukaryotic plasma membranes in providing protection against melittin-induced bilayer lysis. Biochemistry 42:1101-1108. - PubMed
-
- Andres, E., and J. L. Dimarcq. 2005. Clinical development of antimicrobial peptides. Int. J. Antimicrob. Agents 25:448-449. - PubMed
-
- Balaban, N., Y. Gov, A. Giacometti, O. Cirioni, R. Ghiselli, F. Mocchegiani, F. Orlando, G. D'Amato, V. Saba, G. Scalise, S. Bernes, and A. Mor. 2004. A chimeric peptide composed of a dermaseptin derivative and an RNA III-inhibiting peptide prevents graft-associated infections by antibiotic-resistant staphylococci. Antimicrob. Agents Chemother. 48:2544-2550. - PMC - PubMed
-
- Blondelle, S. E., and R. A. Houghten. 1991. Hemolytic and antimicrobial activities of the twenty-four individual omission analogues of melittin. Biochemistry 30:4671-4678. - PubMed
-
- Blondelle, S. E., and K. Lohner. 2000. Combinatorial libraries: a tool to design antimicrobial and antifungal peptide analogues having lytic specificities for structure-activity relationship studies. Biopolymers 55:74-87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

