Novel bacterial acetyl coenzyme A carboxylase inhibitors with antibiotic efficacy in vivo
- PMID: 16870762
- PMCID: PMC1538663
- DOI: 10.1128/AAC.00012-06
Novel bacterial acetyl coenzyme A carboxylase inhibitors with antibiotic efficacy in vivo
Abstract
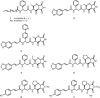
The pseudopeptide pyrrolidinedione antibiotics, such as moiramide B, have recently been discovered to target the multisubunit acetyl coenzyme A (acetyl-CoA) carboxylases of bacteria. In this paper, we describe synthetic variations of each moiety of the modularly composed pyrrolidinediones, providing insight into structure-activity relationships of biochemical target activity, in vitro potency, and in vivo efficacy. The novel derivatives showed highly improved activities against gram-positive bacteria compared to those of previously reported variants. The compounds exhibited a MIC(90) value of 0.1 microg/ml against a broad spectrum of Staphylococcus aureus clinical isolates. No cross-resistance to antibiotics currently used in clinical practice was observed. Resistance mutations induced by pyrrolidinediones are exclusively located in the carboxyltransferase subunits of the bacterial acetyl-CoA carboxylase, indicating the identical mechanisms of action of all derivatives tested. Improvement of the physicochemical profile was achieved by salt formation, leading to aqueous solubilities of up to 5 g/liter. For the first time, the in vitro activity of this compound class was compared with its in vivo efficacy, demonstrating a path from compounds weakly active in vivo to agents with significant efficacy. In a murine model of S. aureus sepsis, the 100% effective dose of the best compound reported was 25 mg/kg of body weight, only fourfold higher than that of the comparator molecule linezolid. The obvious improvements achieved by chemical derivatization reflect the potential of this novel antibiotic compound class for future therapy.
Figures

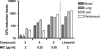

References
-
- Bilder, P., S. Lightle, G. Bainbridge, J. Ohren, B. Finzel, F. Sun, S. Holley, L. Al-Kassim, C. Spessard, M. Melnick, M. Newcomer, and G. L. Waldrop. 2006. The structure of the carboxyltransferase component of acetyl-CoA carboxylase reveals a zinc-binding motif unique to the bacterial enzyme. Biochemistry 45:1712-1722. - PubMed
-
- Broetz-Oesterhelt, H., D. Beyer, H. P. Kroll, R. Endermann, C. Ladel, W. Schroeder, B. Hinzen, S. Raddatz, H. Paulsen, K. Henninger, J. E. Bandow, H. G. Sahl, and H. Labischinski. 2005. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 11:1082-1087. - PubMed
-
- Cronan, J. E., Jr., and G. L. Waldrop. 2002. Multi-subunit acetyl-CoA carboxylases. Prog. Lipid Res. 41:407-435. - PubMed
-
- Davies, S. G., and D. J. Dixon. 1998. Asymmetric syntheses of moiramide B and andrimid. J. Chem. Soc. Perkin 1 17:2635-2644.
-
- Fredenhagen, A., S. Y. Tamura, P. T. M. Kenny, H. Komura, Y. Naya, K. Nakanishi, K. Nishiyama, M. Sugiura, and H. Kita. 1987. Andrimid, a new peptide antibiotic produced by an intracellular bacterial symbiont isolated from a brown planthopper. J. Am. Chem. Soc. 109:4409-4411.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

