Randomised trial of chloroquine/sulphadoxine-pyrimethamine in Gambian children with malaria: impact against multidrug-resistant P. falciparum
- PMID: 16871319
- PMCID: PMC1513406
- DOI: 10.1371/journal.pctr.0010014
Randomised trial of chloroquine/sulphadoxine-pyrimethamine in Gambian children with malaria: impact against multidrug-resistant P. falciparum
Abstract
Objectives: In the Gambia, the combination of chloroquine (CQ) and sulphadoxine-pyrimethamine (SP) has replaced CQ monotherapy for treatment of malaria caused by Plasmodium falciparum. We measured the efficacy of the combination CQ/SP, and the prevalence of parasites carrying alleles associated with resistance to CQ or SP.
Design: We conducted a single-blind, randomised, controlled trial to compare the efficacy of CQ/SP to that of SP or CQ alone.
Setting: The study took place in the town of Farafenni and surrounding villages in the Gambia.
Participants: Participants were children aged 12 mo to 10 y presenting as outpatients with uncomplicated P. falciparum malaria.
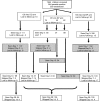
Interventions: 500 children were randomised to receive CQ, SP, or CQ/SP as supervised treatment and actively followed over 28 d.
Outcome measures: Primary outcome was parasitaemia at any time during follow-up. Secondary outcomes were PCR-confirmed recrudescent infections among treatment failures, and clinical failure requiring rescue medication by day 28. Pretreatment parasite isolates from 161 patients were tested for the presence of resistance-associated genetic markers.
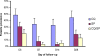
Results: The prevalence of parasitological failure by day 28 for the CQ group was 60.3%, compared to 17.6% for SP (odds ratio [OR], 0.106; 95% confidence interval [CI], 0.057-0.194; p < 0.001) and 13.9% for CQ/SP (OR versus CQ, 0.140; 95% CI, 0.078-0.250; p < 0.001). There was no difference between the SP and CQ/SP groups (OR, 1.324; 95% CI, 0.705-2.50). The projected prevalence of PCR-corrected treatment failure was 30.2, 6.06, and 3.94% in the CQ, SP, and CQ/SP groups, respectively. The pfdhfr-triple mutant and pfdhps-437G mutation were common, with prevalences of 67.4 and 51.2%, respectively. Pretreatment carriage of pfdhps-437G and of multidrug-resistant parasite genotypes was associated with treatment failure in the SP group, but not in the CQ or CQ/SP groups.
Conclusions: The combination of CQ/SP was an efficacious treatment for uncomplicated malaria in Gambian children in this study, but the frequent occurrence of multidrug-resistant parasites suggests that this observed efficacy is not sustainable.
Conflict of interest statement
Figures

References
-
- World Health Organization [WHO] Technical Report Series Number 839. Geneva: World Health Organization; 1993. Implementation of the global malaria control strategy. - PubMed
-
- World Health Organization [WHO], United Nations Children's Fund [UNICEF] Africa Malaria Report 2003. World Health Organization; Geneva: 2003. (WHO/CDS/MAL/2003.1093). Available: http://www.rbm.who.int/amd2003/amr2003/amr_toc.htm. Accessed: 12 February 2005.
-
- Greenwood BM, Bradley AK, Byass P, Greenwood AM, Menon A, et al. Evaluation of a primary health care programme in The Gambia. II. Its impact on mortality and morbidity in young children. J Trop Med Hyg. 1990;93:87–97. - PubMed
-
- Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis. 2001;184:770–776. - PubMed
-
- Müller O, Boele van Hensbroek M, Jaffa S, Drakeley C, Okorie C, et al. A randomized trial of chloroquine, amodiaquine and pyrimethamine-sulfadoxine in Gambian children with uncomplicated malaria. Trop Med Int Health. 1996;1:124–132. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

