Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma
- PMID: 16871333
- PMCID: PMC1488900
- DOI: 10.1371/journal.pctr.0010011
Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma
Abstract
Objectives: The respiratory pathogen Chlamydia pneumoniae (C. pneumoniae) produces acute and chronic lung infections and is associated with asthma. Evidence for effectiveness of antichlamydial antibiotics in asthma is limited. The primary objective of this pilot study was to investigate the feasibility of performing an asthma clinical trial in practice settings where most asthma is encountered and managed. The secondary objectives were to investigate (1) whether azithromycin treatment would affect any asthma outcomes and (2) whether C. pneumoniae serology would be related to outcomes. This report presents the secondary results.
Design: Randomized, placebo-controlled, blinded (participants, physicians, study personnel, data analysts), allocation-concealed parallel group clinical trial.
Setting: Community-based health-care settings located in four states and one Canadian province.
Participants: Adults with stable, persistent asthma.
Interventions: Azithromycin (six weekly doses) or identical matching placebo, plus usual community care.
Outcome measures: Juniper Asthma Quality of Life Questionnaire (Juniper AQLQ), symptom, and medication changes from baseline (pretreatment) to 3 mo posttreatment (follow-up); C. pneumoniae IgG and IgA antibodies at baseline and follow-up.
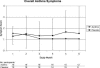
Results: Juniper AQLQ improved by 0.25 (95% confidence interval; -0.3, 0.8) units, overall asthma symptoms improved by 0.68 (0.1, 1.3) units, and rescue inhaler use decreased by 0.59 (-0.5, 1.6) daily administrations in azithromycin-treated compared to placebo-treated participants. Baseline IgA antibodies were positively associated with worsening overall asthma symptoms at follow-up (p = 0.04), but IgG was not (p = 0.63). Overall asthma symptom improvement attributable to azithromycin was 28% in high IgA participants versus 12% in low IgA participants (p for interaction = 0.27).
Conclusions: Azithromycin did not improve Juniper AQLQ but appeared to improve overall asthma symptoms. Larger community-based trials of antichlamydial antibiotics for asthma are warranted.
Conflict of interest statement
Figures

References
-
- Grayston JT, Campbell LA, Kuo C-C, Mordhorst CH, Saikku P, et al. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis. 1990;161:618–625. - PubMed
-
- Hahn DL, Dodge R, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266:225–230. - PubMed
-
- Hahn DL, Golubjatnikov R. Asthma and chlamydial infection: A case series. J Fam Pract. 1994;38:589–595. - PubMed
-
- Björnsson E, Helm E, Janson C, Fridell E, Boman G. Serology of chlamydia in relation to asthma and bronchial hyperresponsiveness. Scand J Infect Dis. 1996;28:63–69. - PubMed
-
- Gencay M, Rüdiger JJ, Tamm M, Solér M, Perruchoud AP, et al. Increased frequency of Chlamydia pneumoniae antibodies in patients with asthma. Am J Respir Crit Care Med. 2001;163:1097–1100. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

