Molecular characterization of retinal stem cells and their niches in adult zebrafish
- PMID: 16872490
- PMCID: PMC1564002
- DOI: 10.1186/1471-213X-6-36
Molecular characterization of retinal stem cells and their niches in adult zebrafish
Abstract
Background: The persistence in adult teleost fish of retinal stem cells that exhibit all of the features of true 'adult stem cells'--self-renewal, multipotency, and the capacity to respond to injury by mitotic activation with the ability to regenerate differentiated tissues--has been known for several decades. However, the specialized cellular and molecular characteristics of these adult retinal stem cells and the microenvironmental niches that support their maintenance in the differentiated retina and regulate their activity during growth and regeneration have not yet been elucidated.
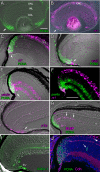
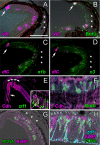
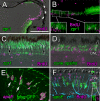
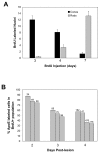
Results: Our data show that the zebrafish retina has two kinds of specialized niches that sustain retinal stem cells: 1) a neuroepithelial germinal zone at the interface between neural retina and ciliary epithelium, called the ciliary marginal zone (CMZ), a continuous annulus around the retinal circumference, and 2) the microenvironment around some Müller glia in the differentiated retina. In the uninjured retina, scattered Müller glia (more frequently those in peripheral retina) are associated with clusters of proliferating retinal progenitors that are restricted to the rod photoreceptor lineage, but following injury, the Müller-associated retinal progenitors can function as multipotent retinal stem cells to regenerate other types of retinal neurons. The CMZ has several features in common with the neurogenic niches in the adult mammalian brain, including access to the apical epithelial surface and a close association with blood vessels. Müller glia in the teleost retina have a complex response to local injury that includes some features of reactive gliosis (up-regulation of glial fibrillary acidic protein, GFAP, and re-entry into the cell cycle) together with dedifferentiation and re-acquisition of phenotypic and molecular characteristics of multipotent retinal progenitors in the CMZ (diffuse distribution of N-cadherin, activation of Notch-Delta signaling, and expression of rx1, vsx2/Chx10, and pax6a) along with characteristics associated with radial glia (expression of brain lipid binding protein, BLBP). We also describe a novel specific marker for Müller glia, apoE.
Conclusion: The stem cell niches that support multi-lineage retinal progenitors in the intact, growing and regenerating teleost retina have properties characteristic of neuroepithelia and neurogenic radial glia. The regenerative capacity of the adult zebrafish retina with its ability to replace lost retinal neurons provides an opportunity to discover the molecular regulators that lead to functional repair of damaged neural tissue.
Figures



References
-
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. - PubMed
-
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. - PubMed
-
- Mori T, Buffo A, Götz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. - PubMed
-
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. - PubMed
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

