Increased large conductance calcium-activated potassium (BK) channel expression accompanied by STREX variant downregulation in the developing mouse CNS
- PMID: 16872513
- PMCID: PMC1562363
- DOI: 10.1186/1471-213X-6-37
Increased large conductance calcium-activated potassium (BK) channel expression accompanied by STREX variant downregulation in the developing mouse CNS
Abstract
Background: Large conductance calcium- and voltage activated potassium (BK) channels are important determinants of neuronal excitability through effects on action potential duration, frequency and synaptic efficacy. The pore- forming subunits are encoded by a single gene, KCNMA1, which undergoes extensive alternative pre mRNA splicing. Different splice variants can confer distinct properties on BK channels. For example, insertion of the 58 amino acid stress-regulated exon (STREX) insert, that is conserved throughout vertebrate evolution, encodes channels with distinct calcium sensitivity and regulation by diverse signalling pathways compared to the insertless (ZERO) variant. Thus, expression of distinct splice variants may allow cells to differentially shape their electrical properties during development. However, whether differential splicing of BK channel variants occurs during development of the mammalian CNS has not been examined.
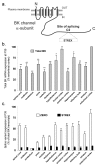
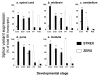
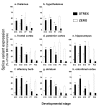
Results: Using quantitative real-time polymerase chain reaction (RT-PCR) Taqmantrade mark assays, we demonstrate that total BK channel transcripts are up regulated throughout the murine CNS during embryonic and postnatal development with regional variation in transcript levels. This upregulation is associated with a decrease in STREX variant mRNA expression and an upregulation in ZERO variant expression.
Conclusion: As BK channel splice variants encode channels with distinct functional properties the switch in splicing from the STREX phenotype to ZERO phenotype during embryonic and postnatal CNS development may provide a mechanism to allow BK channels to control distinct functions at different times of mammalian brain development.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

