Identification of new participants in the rainbow trout (Oncorhynchus mykiss) oocyte maturation and ovulation processes using cDNA microarrays
- PMID: 16872517
- PMCID: PMC1570352
- DOI: 10.1186/1477-7827-4-39
Identification of new participants in the rainbow trout (Oncorhynchus mykiss) oocyte maturation and ovulation processes using cDNA microarrays
Abstract
Background: The hormonal control of oocyte maturation and ovulation as well as the molecular mechanisms of nuclear maturation have been thoroughly studied in fish. In contrast, the other molecular events occurring in the ovary during post-vitellogenesis have received far less attention.
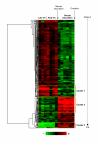
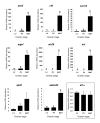
Methods: Nylon microarrays displaying 9152 rainbow trout cDNAs were hybridized using RNA samples originating from ovarian tissue collected during late vitellogenesis, post-vitellogenesis and oocyte maturation. Differentially expressed genes were identified using a statistical analysis. A supervised clustering analysis was performed using only differentially expressed genes in order to identify gene clusters exhibiting similar expression profiles. In addition, specific genes were selected and their preovulatory ovarian expression was analyzed using real-time PCR.
Results: From the statistical analysis, 310 differentially expressed genes were identified. Among those genes, 90 were up-regulated at the time of oocyte maturation while 220 exhibited an opposite pattern. After clustering analysis, 90 clones belonging to 3 gene clusters exhibiting the most remarkable expression patterns were kept for further analysis. Using real-time PCR analysis, we observed a strong up-regulation of ion and water transport genes such as aquaporin 4 (aqp4) and pendrin (slc26). In addition, a dramatic up-regulation of vasotocin (avt) gene was observed. Furthermore, angiotensin-converting-enzyme 2 (ace2), coagulation factor V (cf5), adam 22, and the chemokine cxcl14 genes exhibited a sharp up-regulation at the time of oocyte maturation. Finally, ovarian aromatase (cyp19a1) exhibited a dramatic down-regulation over the post-vitellogenic period while a down-regulation of Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (cmah) was observed at the time of oocyte maturation.
Conclusion: We showed the over or under expression of more that 300 genes, most of them being previously unstudied or unknown in the fish preovulatory ovary. Our data confirmed the down-regulation of estrogen synthesis genes during the preovulatory period. In addition, the strong up-regulation of aqp4 and slc26 genes prior to ovulation suggests their participation in the oocyte hydration process occurring at that time. Furthermore, among the most up-regulated clones, several genes such as cxcl14, ace2, adam22, cf5 have pro-inflammatory, vasodilatory, proteolytics and coagulatory functions. The identity and expression patterns of those genes support the theory comparing ovulation to an inflammatory-like reaction.
Figures
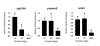
References
-
- Goetz FW. Hormonal control of oocyte final maturation and ovulation in fishes. In: Hoar WS, Randall DJ, Donaldson EM, editor. Fish Physiology. New York: Academic Press; 1983. pp. 117–170.
-
- Goetz FW, Garczynski M. The ovarian regulation of ovulation in teleost fish. Fish Physiology and Biochemistry. 1997;17:33–38. doi: 10.1023/A:1007765902327. - DOI
-
- Jalabert B, Fostier A, Breton B, Weil C. Oocyte Maturation in Vertebrates. In: Pang P, Schreibman M, editor. Vertebrate Endocrinology, Fundamentals and Biomedical Implications. New York: Academic Press; 1991. pp. 23–90.
-
- Patino R, Sullivan CV. Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiology and Biochemistry. 2002;26:57–70. doi: 10.1023/A:1023311613987. - DOI
-
- Yamashita M, Mita K, Yoshida N, Kondo T. Molecular mechanisms of the initiation of oocyte maturation: general and species-specific aspects. Prog Cell Cycle Res. 2000;4:115–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

