Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene
- PMID: 16872525
- PMCID: PMC1779392
- DOI: 10.1186/ar2024
Prostaglandin E2 synthesis in cartilage explants under compression: mPGES-1 is a mechanosensitive gene
Abstract
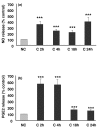
Knee osteoarthritis (OA) results, at least in part, from overloading and inflammation leading to cartilage degradation. Prostaglandin E2 (PGE2) is one of the main catabolic factors involved in OA. Its synthesis is the result of cyclooxygenase (COX) and prostaglandin E synthase (PGES) activities whereas NAD+-dependent 15 hydroxy prostaglandin dehydrogenase (15-PGDH) is the key enzyme implicated in the catabolism of PGE2. For both COX and PGES, three isoforms have been described: in cartilage, COX-1 and cytosolic PGES are constitutively expressed whereas COX-2 and microsomal PGES type 1 (mPGES-1) are inducible in an inflammatory context. COX-3 (a variant of COX-1) and mPGES-2 have been recently cloned but little is known about their expression and regulation in cartilage, as is also the case for 15-PGDH. We investigated the regulation of the genes encoding COX and PGES isoforms during mechanical stress applied to cartilage explants. Mouse cartilage explants were subjected to compression (0.5 Hz, 1 MPa) for 2 to 24 hours. After determination of the amount of PGE2 released in the media (enzyme immunoassay), mRNA and proteins were extracted directly from the cartilage explants and analyzed by real-time RT-PCR and western blotting respectively. Mechanical compression of cartilage explants significantly increased PGE2 production in a time-dependent manner. This was not due to the synthesis of IL-1, since pretreatment with interleukin 1 receptor antagonist (IL1-Ra) did not alter the PGE2 synthesis. Interestingly, COX-2 and mPGES-1 mRNA expression significantly increased after 2 hours, in parallel with protein expression, whereas COX-3 and mPGES-2 mRNA expression was not modified. Moreover, we observed a delayed overexpression of 15-PGDH just before the decline of PGE2 synthesis after 18 hours, suggesting that PGE2 synthesis could be altered by the induction of 15-PGDH expression. We conclude that, along with COX-2, dynamic compression induces mPGES-1 mRNA and protein expression in cartilage explants. Thus, the mechanosensitive mPGES-1 enzyme represents a potential therapeutic target in osteoarthritis.
Figures

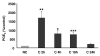



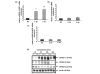
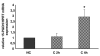
Similar articles
-
Mechanical stress and prostaglandin E2 synthesis in cartilage.Biorheology. 2008;45(3-4):301-20. Biorheology. 2008. PMID: 18836232
-
Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis.Arthritis Res Ther. 2004;6(4):R355-65. doi: 10.1186/ar1195. Epub 2004 Jun 8. Arthritis Res Ther. 2004. PMID: 15225371 Free PMC article.
-
Expression and regulation of microsomal prostaglandin E synthase-1 in human osteoarthritic cartilage and chondrocytes.J Rheumatol. 2005 May;32(5):887-95. J Rheumatol. 2005. PMID: 15868626
-
Prostaglandin E2 as a mediator of fever: synthesis and catabolism.Front Biosci. 2004 May 1;9:1977-93. doi: 10.2741/1383. Front Biosci. 2004. PMID: 14977603 Review.
-
[Prostaglandin E2 synthases].Nihon Yakurigaku Zasshi. 2002 Dec;120(6):373-8. doi: 10.1254/fpj.120.373. Nihon Yakurigaku Zasshi. 2002. PMID: 12528468 Review. Japanese.
Cited by
-
Metformin use and the risk of total knee replacement among diabetic patients: a propensity-score-matched retrospective cohort study.Sci Rep. 2022 Jul 7;12(1):11571. doi: 10.1038/s41598-022-15871-7. Sci Rep. 2022. PMID: 35798867 Free PMC article.
-
Investigating conversion of mechanical force into biochemical signaling in three-dimensional chondrocyte cultures.Nat Protoc. 2009;4(6):928-38. doi: 10.1038/nprot.2009.63. Epub 2009 May 28. Nat Protoc. 2009. PMID: 19478808
-
Effects of Inflammation on Multiscale Biomechanical Properties of Cartilaginous Cells and Tissues.ACS Biomater Sci Eng. 2017 Nov 13;3(11):2644-2656. doi: 10.1021/acsbiomaterials.6b00671. Epub 2017 Jan 24. ACS Biomater Sci Eng. 2017. PMID: 29152560 Free PMC article. Review.
-
Genome-wide association scan identifies a prostaglandin-endoperoxide synthase 2 variant involved in risk of knee osteoarthritis.Am J Hum Genet. 2008 Jun;82(6):1231-40. doi: 10.1016/j.ajhg.2008.04.006. Epub 2008 May 8. Am J Hum Genet. 2008. PMID: 18471798 Free PMC article.
-
Dynamic compression counteracts IL-1beta induced inducible nitric oxide synthase and cyclo-oxygenase-2 expression in chondrocyte/agarose constructs.Arthritis Res Ther. 2008;10(2):R35. doi: 10.1186/ar2389. Epub 2008 Mar 18. Arthritis Res Ther. 2008. PMID: 18348730 Free PMC article.
References
-
- Centers for Disease Control and Prevention Arthritis prevalence and activity limitations – United States, 1990. MMWR Morb Mortal Wkly Rep. 1994;43:433–438. - PubMed
-
- Islam N, Haqqi TM, Jepsen KJ, Kraay M, Welter JF, Goldberg VM, Malemud CJ. Hydrostatic pressure induces apoptosis in human chondrocytes from osteoarthritic cartilage through up-regulation of tumor necrosis factor-alpha, inducible nitric oxide synthase, p53, c-myc, and bax-alpha, and suppression of bcl-2. J Cell Biochem. 2002;87:266–278. doi: 10.1002/jcb.10317. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

