Darwin at the molecular scale: selection and variance in electron tunnelling proteins including cytochrome c oxidase
- PMID: 16873117
- PMCID: PMC1647310
- DOI: 10.1098/rstb.2006.1868
Darwin at the molecular scale: selection and variance in electron tunnelling proteins including cytochrome c oxidase
Abstract
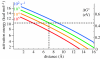
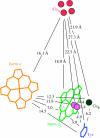
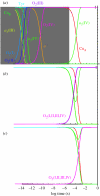
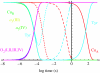
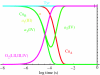
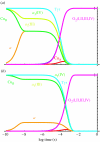
Biological electron transfer is designed to connect catalytic clusters by chains of redox cofactors. A review of the characterized natural redox proteins with a critical eye for molecular scale measurement of variation and selection related to physiological function shows no statistically significant differences in the protein medium lying between cofactors engaged in physiologically beneficial or detrimental electron transfer. Instead, control of electron tunnelling over long distances relies overwhelmingly on less than 14 A spacing between the cofactors in a chain. Near catalytic clusters, shorter distances (commonly less than 7 A) appear to be selected to generate tunnelling frequencies sufficiently high to scale the barriers of multi-electron, bond-forming/-breaking catalysis at physiological rates. We illustrate this behaviour in a tunnelling network analysis of cytochrome c oxidase. In order to surmount the large, thermally activated, adiabatic barriers in the 5-10 kcal mol-1 range expected for H+ motion and O2 reduction at the binuclear centre of oxidase on the 10(3)-10(5) s-1 time-scale of respiration, electron access with a tunnelling frequency of 10(9) or 10(10) s-1 is required. This is provided by selecting closely placed redox centres, such as haem a (6.9 A) or tyrosine (4.9 A). A corollary is that more distantly placed redox centres, such as CuA, cannot rapidly scale the catalytic site barrier, but must send their electrons through more closely placed centres, avoiding direct short circuits that might circumvent proton pumping coupled to haems a to a3 electron transfer. The selection of distances and energetic barriers directs electron transfer from CuA to haem a rather than a3, without any need for delicate engineering of the protein medium to 'hard wire' electron transfer. Indeed, an examination of a large number of oxidoreductases provides no evidence of such naturally selected wiring of electron tunnelling pathways.
Figures
Similar articles
-
Natural engineering principles of electron tunnelling in biological oxidation-reduction.Nature. 1999 Nov 4;402(6757):47-52. doi: 10.1038/46972. Nature. 1999. PMID: 10573417
-
Connecting CuA with metal centers of heme a, heme a3, CuB and Zn by pathways with hydrogen bond as the bridging element in cytochrome c oxidase.Biochem Biophys Res Commun. 2019 Mar 5;510(2):261-265. doi: 10.1016/j.bbrc.2019.01.083. Epub 2019 Jan 25. Biochem Biophys Res Commun. 2019. PMID: 30686530
-
Time-resolved generation of membrane potential by ba3 cytochrome c oxidase from Thermus thermophilus coupled to single electron injection into the O and OH states.Biochim Biophys Acta Bioenerg. 2017 Nov;1858(11):915-926. doi: 10.1016/j.bbabio.2017.08.007. Epub 2017 Aug 12. Biochim Biophys Acta Bioenerg. 2017. PMID: 28807731
-
Proton-controlled electron transfer in cytochrome c oxidase: functional role of the pathways through Glu 286 and Lys 362.Acta Physiol Scand Suppl. 1998 Aug;643:7-16. Acta Physiol Scand Suppl. 1998. PMID: 9789542 Review.
-
Measurement of cytochrome oxidase and mitochondrial energetics by near-infrared spectroscopy.Philos Trans R Soc Lond B Biol Sci. 1997 Jun 29;352(1354):669-76. doi: 10.1098/rstb.1997.0048. Philos Trans R Soc Lond B Biol Sci. 1997. PMID: 9232854 Free PMC article. Review.
Cited by
-
Multistationary and oscillatory modes of free radicals generation by the mitochondrial respiratory chain revealed by a bifurcation analysis.PLoS Comput Biol. 2012;8(9):e1002700. doi: 10.1371/journal.pcbi.1002700. Epub 2012 Sep 20. PLoS Comput Biol. 2012. PMID: 23028295 Free PMC article.
-
Engineering Synthetic Electron Transfer Chains from Metallopeptide Membranes.Inorg Chem. 2024 Feb 12;63(6):2899-2908. doi: 10.1021/acs.inorgchem.3c02861. Epub 2023 Dec 21. Inorg Chem. 2024. PMID: 38127051 Free PMC article.
-
A connecting hinge represses the activity of endothelial nitric oxide synthase.Proc Natl Acad Sci U S A. 2007 May 29;104(22):9254-9. doi: 10.1073/pnas.0700332104. Epub 2007 May 21. Proc Natl Acad Sci U S A. 2007. PMID: 17517617 Free PMC article.
-
D1-arginine257 mutants (R257E, K, and Q) of Chlamydomonas reinhardtii have a lowered QB redox potential: analysis of thermoluminescence and fluorescence measurements.Photosynth Res. 2008 Oct-Dec;98(1-3):449-68. doi: 10.1007/s11120-008-9351-9. Epub 2008 Sep 6. Photosynth Res. 2008. PMID: 18777103 Free PMC article.
-
Inter-monomer electron transfer is too slow to compete with monomeric turnover in bc(1) complex.Biochim Biophys Acta. 2012 Jul;1817(7):1053-62. doi: 10.1016/j.bbabio.2012.03.012. Epub 2012 Mar 17. Biochim Biophys Acta. 2012. PMID: 22465023 Free PMC article.
References
-
- Adelroth P, Brzezinski P, Malmstrom B.G. Internal electron transfer in cytochrome c oxidase from Rhodobacter sphaeroides. Biochemistry. 1995;34:2844–2849. doi:10.1021/bi00009a014 - DOI - PubMed
-
- Adelroth P, Ek M, Brzezinski P. Factors determining electron-transfer rates in cytochrome c oxidase: investigation of the oxygen reaction in the R. sphaeroides enzyme. Biochim. Biophys. Acta. 1998;1367:107–117. doi:10.1016/S0005-2728(98)00142-X - DOI - PubMed
-
- Babcock G.T, Callahan P.M. Redox-linked hydrogen-bond strength changes in cytochrome a: implications for a cytochrome oxidase proton pump. Biochemistry. 1983;22:2314–2319. doi:10.1021/bi00279a002 - DOI - PubMed
-
- Babcock G.T, Wikstrom M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–309. doi:10.1038/356301a0 - DOI - PubMed
-
- Beratan D.N, Betts J.N, Onuchic J.N. Tunneling pathway and redox-state-dependent electronic couplings at nearly fixed distance in electron-transfer proteins. J. Phys. Chem. 1992;96:2852–2855. doi:10.1021/j100186a014 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

