Hydride transfer catalysed by Escherichia coli and Bacillus subtilis dihydrofolate reductase: coupled motions and distal mutations
- PMID: 16873124
- PMCID: PMC1647314
- DOI: 10.1098/rstb.2006.1869
Hydride transfer catalysed by Escherichia coli and Bacillus subtilis dihydrofolate reductase: coupled motions and distal mutations
Abstract
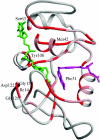
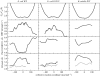
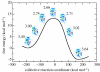
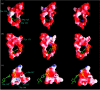
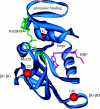
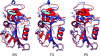
This paper reviews the results from hybrid quantum/classical molecular dynamics simulations of the hydride transfer reaction catalysed by wild-type (WT) and mutant Escherichia coli and WT Bacillus subtilis dihydrofolate reductase (DHFR). Nuclear quantum effects such as zero point energy and hydrogen tunnelling are significant in these reactions and substantially decrease the free energy barrier. The donor-acceptor distance decreases to ca 2.7 A at transition-state configurations to enable the hydride transfer. A network of coupled motions representing conformational changes along the collective reaction coordinate facilitates the hydride transfer reaction by decreasing the donor-acceptor distance and providing a favourable geometric and electrostatic environment. Recent single-molecule experiments confirm that at least some of these thermally averaged equilibrium conformational changes occur on the millisecond time-scale of the hydride transfer. Distal mutations can lead to non-local structural changes and significantly impact the probability of sampling configurations conducive to the hydride transfer, thereby altering the free-energy barrier and the rate of hydride transfer. E. coli and B. subtilis DHFR enzymes, which have similar tertiary structures and hydride transfer rates with 44% sequence identity, exhibit both similarities and differences in the equilibrium motions and conformational changes correlated to hydride transfer, suggesting a balance of conservation and flexibility across species.
Figures
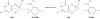
Similar articles
-
Comparison of coupled motions in Escherichia coli and Bacillus subtilis dihydrofolate reductase.J Phys Chem B. 2006 May 25;110(20):10130-8. doi: 10.1021/jp0605956. J Phys Chem B. 2006. PMID: 16706474
-
Impact of distal mutations on the network of coupled motions correlated to hydride transfer in dihydrofolate reductase.Proc Natl Acad Sci U S A. 2005 May 10;102(19):6807-12. doi: 10.1073/pnas.0408343102. Epub 2005 Apr 5. Proc Natl Acad Sci U S A. 2005. PMID: 15811945 Free PMC article.
-
Freezing a single distal motion in dihydrofolate reductase.J Phys Chem B. 2006 Feb 9;110(5):2435-41. doi: 10.1021/jp056939u. J Phys Chem B. 2006. PMID: 16471835
-
Catalytic efficiency of enzymes: a theoretical analysis.Biochemistry. 2013 Mar 26;52(12):2012-20. doi: 10.1021/bi301515j. Epub 2012 Dec 20. Biochemistry. 2013. PMID: 23240765 Free PMC article. Review.
-
Multiple intermediates, diverse conformations, and cooperative conformational changes underlie the catalytic hydride transfer reaction of dihydrofolate reductase.Top Curr Chem. 2013;337:165-87. doi: 10.1007/128_2012_408. Top Curr Chem. 2013. PMID: 23420416 Free PMC article. Review.
Cited by
-
Evolution Conserves the Network of Coupled Residues in Dihydrofolate Reductase.Biochemistry. 2019 Sep 17;58(37):3861-3868. doi: 10.1021/acs.biochem.9b00460. Epub 2019 Aug 30. Biochemistry. 2019. PMID: 31423766 Free PMC article.
-
Structural and mechanistic insights into Streptococcus pneumoniae NADPH oxidase.Nat Struct Mol Biol. 2024 Nov;31(11):1769-1777. doi: 10.1038/s41594-024-01348-w. Epub 2024 Jul 22. Nat Struct Mol Biol. 2024. PMID: 39039317 Free PMC article.
-
Exploring the molecular origins of protein dynamics in the active site of human carbonic anhydrase II.J Phys Chem B. 2009 Aug 20;113(33):11505-10. doi: 10.1021/jp901321m. J Phys Chem B. 2009. PMID: 19637848 Free PMC article.
-
Second-Shell Residues Contribute to Catalysis by Predominately Preorganizing the Apo State in PafA.J Am Chem Soc. 2023 May 24;145(20):11333-11347. doi: 10.1021/jacs.3c02423. Epub 2023 May 12. J Am Chem Soc. 2023. PMID: 37172218 Free PMC article.
-
X-ray-driven chemistry and conformational heterogeneity in atomic resolution crystal structures of bacterial dihydrofolate reductases.bioRxiv [Preprint]. 2023 Nov 8:2023.11.07.566054. doi: 10.1101/2023.11.07.566054. bioRxiv. 2023. PMID: 37986818 Free PMC article. Preprint.
References
-
- Agarwal P.K, Billeter S.R, Hammes-Schiffer S. Nuclear quantum effects and enzyme dynamics in dihydrofolate reductase catalysis. J. Phys. Chem. B. 2002a;106:3283–3293. doi:10.1021/jp020190v - DOI
-
- Agarwal P.K, Billeter S.R, Rajagopalan P.T.R, Benkovic S.J, Hammes-Schiffer S. Network of coupled promoting motions in enzyme catalysis. Proc. Natl Acad. Sci. USA. 2002b;99:2794–2799. doi:10.1073/pnas.052005999 - DOI - PMC - PubMed
-
- Anderson J.B. Statistical theories of chemical reactions. Distributions in the transition region. J. Chem. Phys. 1973;58:4684–4692. doi:10.1063/1.1679032 - DOI
-
- Antikainen N.M, Smiley R.D, Benkovic S.J, Hammes G.G. Conformation coupled enzyme catalysis: single molecule and transient kinetics investigation of dihydrofolate reductase. Biochemistry. 2005;44:16 835–16 843. - PubMed
-
- Benkovic S.J, Hammes-Schiffer S. A perspective on enzyme catalysis. Science. 2003;301:1196–1202. doi:10.1126/science.1085515 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

