Isolation and characterization of monoclonal antibodies which neutralize human metapneumovirus in vitro and in vivo
- PMID: 16873237
- PMCID: PMC1563801
- DOI: 10.1128/JVI.00318-06
Isolation and characterization of monoclonal antibodies which neutralize human metapneumovirus in vitro and in vivo
Abstract
Human metapneumovirus (hMPV) is a recently described member of the Paramyxoviridae family/Pneumovirinae subfamily and shares many common features with respiratory syncytial virus (RSV), another member of the same subfamily. hMPV causes respiratory tract illnesses that, similar to human RSV, occur predominantly during the winter months and have symptoms that range from mild to severe cough, bronchiolitis, and pneumonia. Like RSV, the hMPV virus can be subdivided into two genetic subgroups, A and B. With RSV, a single monoclonal antibody directed at the fusion (F) protein can prevent severe lower respiratory tract RSV infection. Because of the high level of sequence conservation of the F protein across all the hMPV subgroups, this protein is likely to be the preferred antigenic target for the generation of cross-subgroup neutralizing antibodies. Here we describe the generation of a panel of neutralizing monoclonal antibodies that bind to the hMPV F protein. A subset of these antibodies has the ability to neutralize prototypic strains of both the A and B hMPV subgroups in vitro. Two of these antibodies exhibited high-affinity binding to the F protein and were shown to protect hamsters against infection with hMPV. The data suggest that a monoclonal antibody could be used prophylactically to prevent lower respiratory tract disease caused by hMPV.
Figures

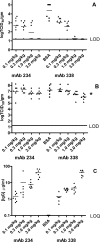
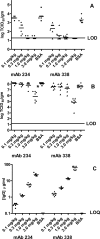
 ) and <0.0001 (#). IgG, immunoglobulin G.
) and <0.0001 (#). IgG, immunoglobulin G.Similar articles
-
A Potent Neutralizing Site III-Specific Human Antibody Neutralizes Human Metapneumovirus In Vivo.J Virol. 2019 Sep 12;93(19):e00342-19. doi: 10.1128/JVI.00342-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31292250 Free PMC article.
-
A broadly neutralizing human monoclonal antibody exhibits in vivo efficacy against both human metapneumovirus and respiratory syncytial virus.J Infect Dis. 2015 Jan 15;211(2):216-25. doi: 10.1093/infdis/jiu307. Epub 2014 May 26. J Infect Dis. 2015. PMID: 24864121 Free PMC article.
-
Identification of antibody neutralization epitopes on the fusion protein of human metapneumovirus.J Gen Virol. 2008 Dec;89(Pt 12):3113-3118. doi: 10.1099/vir.0.2008/005199-0. J Gen Virol. 2008. PMID: 19008400 Free PMC article.
-
Human Metapneumovirus.Microbiol Spectr. 2014 Oct;2(5). doi: 10.1128/microbiolspec.AID-0020-2014. Microbiol Spectr. 2014. PMID: 26104361 Review.
-
Vaccination approaches to combat human metapneumovirus lower respiratory tract infections.J Clin Virol. 2008 Jan;41(1):49-52. doi: 10.1016/j.jcv.2007.10.022. Epub 2007 Dec 4. J Clin Virol. 2008. PMID: 18054841 Review.
Cited by
-
Antibody recognition of the Pneumovirus fusion protein trimer interface.PLoS Pathog. 2020 Oct 9;16(10):e1008942. doi: 10.1371/journal.ppat.1008942. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33035266 Free PMC article.
-
Neonatal Immune Responses to Respiratory Viruses.Front Immunol. 2022 Apr 14;13:863149. doi: 10.3389/fimmu.2022.863149. eCollection 2022. Front Immunol. 2022. PMID: 35493465 Free PMC article. Review.
-
Respiratory Virus Infections in Hematopoietic Cell Transplant Recipients.Front Microbiol. 2019 Jan 9;9:3294. doi: 10.3389/fmicb.2018.03294. eCollection 2018. Front Microbiol. 2019. PMID: 30687278 Free PMC article. Review.
-
Cross-neutralization of four paramyxoviruses by a human monoclonal antibody.Nature. 2013 Sep 19;501(7467):439-43. doi: 10.1038/nature12442. Epub 2013 Aug 18. Nature. 2013. PMID: 23955151
-
Human antibody recognition of antigenic site IV on Pneumovirus fusion proteins.PLoS Pathog. 2018 Feb 22;14(2):e1006837. doi: 10.1371/journal.ppat.1006837. eCollection 2018 Feb. PLoS Pathog. 2018. PMID: 29470533 Free PMC article.
References
-
- Atkins, J. T., P. Karimi, B. H. Morris, G. McDavid, and S. Shim. 2000. Prophylaxis for respiratory syncytial virus with respiratory syncytial virus-immunoglobulin intravenous among preterm infants of thirty-two weeks gestation and less: reduction in incidence, severity of illness and cost. Pediatr. Infect. Dis. 19:138-143. - PubMed
-
- Bebbington, C. R., G. Renner, S. Thomson, D. King, D. Abrams, and G. T. Yarranton. 1992. High level expression of a recombinant antibody from myeloma cells using a glutamine synthetase gene as an amplifiable selectable marker. Bio/Technology 10:169-175. - PubMed
-
- Biacchesi, S., M. H. Skiadopoulos, G. Boivin, C. T. Hanson, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2003. Genetic diversity between human metapneumovirus subgroups. Virology 315:1-9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

