Influenza A virus PB1-F2 protein contributes to viral pathogenesis in mice
- PMID: 16873254
- PMCID: PMC1563817
- DOI: 10.1128/JVI.00415-06
Influenza A virus PB1-F2 protein contributes to viral pathogenesis in mice
Abstract
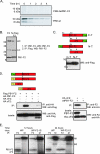
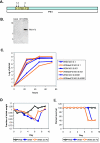
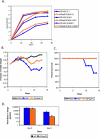
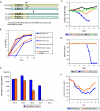
The influenza virus PB1-F2 protein is a novel protein previously shown to be involved in induction of cell death. Here we characterize the expression and the function of the protein within the context of influenza viral infection in tissue culture and a mouse model. We show that the C-terminal region of the protein can be expressed from a downstream initiation codon and is capable of interaction with the full-length protein. Using this knowledge, we generated influenza viruses knocked out for the expression of PB1-F2 protein and its downstream truncation products. Knocking out the PB1-F2 protein had no effect on viral replication in tissue culture but diminished virus pathogenicity and mortality in mice. The viruses replicated to similar levels in mouse lungs by day 3 postinfection, suggesting that the knockout did not impair viral replication. However, while the PB1-F2 knockout viruses were cleared after day 5, the wild-type viruses were detectable in mouse lungs until day 7, implying that expression of PB1-F2 resulted in delayed clearance of the viruses by the host immune system. Based on our findings and on the fact that the PB1 genomic segment was always newly introduced into some pandemic influenza viruses of the last century, we speculate that the PB1-F2 protein plays an important role in pathogenesis of influenza virus infection and may be an important contributor to pathogenicity of pandemic influenza viruses.
Figures
Similar articles
-
Effects of PB1-F2 on the pathogenicity of H1N1 swine influenza virus in mice and pigs.J Gen Virol. 2017 Jan;98(1):31-42. doi: 10.1099/jgv.0.000695. J Gen Virol. 2017. PMID: 28008819 Free PMC article.
-
A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence.PLoS Pathog. 2007 Oct 5;3(10):1414-21. doi: 10.1371/journal.ppat.0030141. PLoS Pathog. 2007. PMID: 17922571 Free PMC article.
-
Emergence of Highly Pathogenic Avian Influenza A(H5N1) Virus PB1-F2 Variants and Their Virulence in BALB/c Mice.J Virol. 2015 Jun;89(11):5835-46. doi: 10.1128/JVI.03137-14. Epub 2015 Mar 18. J Virol. 2015. PMID: 25787281 Free PMC article.
-
Evolution and Virulence of Influenza A Virus Protein PB1-F2.Int J Mol Sci. 2017 Dec 29;19(1):96. doi: 10.3390/ijms19010096. Int J Mol Sci. 2017. PMID: 29286299 Free PMC article. Review.
-
Influenza A virus PB1-F2: a small protein with a big punch.Cell Host Microbe. 2007 Oct 11;2(4):207-9. doi: 10.1016/j.chom.2007.09.010. Cell Host Microbe. 2007. PMID: 18005736 Review.
Cited by
-
Detection of genetic markers related to high pathogenicity in influenza by SERS.Analyst. 2013 Sep 7;138(17):4877-84. doi: 10.1039/c3an00774j. Epub 2013 Jul 8. Analyst. 2013. PMID: 23833767 Free PMC article.
-
Molecular events leading to the creation of a pandemic influenza virus.Indian J Microbiol. 2009 Dec;49(4):332-8. doi: 10.1007/s12088-009-0059-0. Epub 2010 Jan 7. Indian J Microbiol. 2009. PMID: 23100794 Free PMC article.
-
Molecular character of influenza A/H1N1 2009: Implications for spread and control.Indian J Microbiol. 2009 Dec;49(4):339-47. doi: 10.1007/s12088-009-0060-7. Epub 2010 Jan 7. Indian J Microbiol. 2009. PMID: 23100795 Free PMC article.
-
The Influenza Virus Protein PB1-F2 Increases Viral Pathogenesis through Neutrophil Recruitment and NK Cells Inhibition.PLoS One. 2016 Oct 31;11(10):e0165361. doi: 10.1371/journal.pone.0165361. eCollection 2016. PLoS One. 2016. PMID: 27798704 Free PMC article.
-
The role of PB1-F2 in adaptation of high pathogenicity avian influenza virus H7N7 in chickens.Vet Res. 2024 Jan 3;55(1):5. doi: 10.1186/s13567-023-01257-8. Vet Res. 2024. PMID: 38173025 Free PMC article.
References
-
- Chen, W., J. R. Bennink, and J. W. Yewdell. 2003. Systematic search fails to detect immunogenic MHC class-I-restricted determinants encoded by influenza A virus noncoding sequences. Virology 305:50-54. - PubMed
-
- Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. - PubMed
-
- Claas, E. C., J. C. de Jong, R. van Beek, G. F. Rimmelzwaan, and A. D. Osterhaus. 1998. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine 16:977-978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

