PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0
- PMID: 16873256
- PMCID: PMC1563828
- DOI: 10.1128/JVI.00734-06
PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0
Abstract
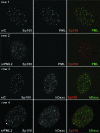
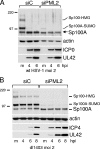
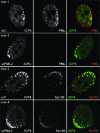
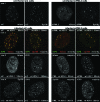
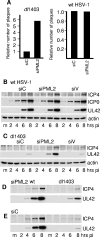
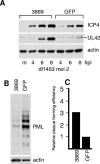
Promyelocytic leukemia (PML) nuclear bodies (also known as ND10) are nuclear substructures that contain several proteins, including PML itself, Sp100, and hDaxx. PML has been implicated in many cellular processes, and ND10 are frequently associated with the replicating genomes of DNA viruses. During herpes simplex virus type 1 (HSV-1) infection, the viral regulatory protein ICP0 localizes to ND10 and induces the degradation of PML, thereby disrupting ND10 and dispersing their constituent proteins. ICP0-null mutant viruses are defective in PML degradation and ND10 disruption, and concomitantly they initiate productive infection very inefficiently. Although these data are consistent with a repressive role for PML and/or ND10 during HSV-1 infection, evidence in support of this hypothesis has been inconclusive. By use of short interfering RNA technology, we demonstrate that depletion of PML increases both gene expression and plaque formation by an ICP0-negative HSV-1 mutant, while having no effect on wild-type HSV-1. We conclude that PML contributes to a cellular antiviral repression mechanism that is countered by the activity of ICP0.
Figures


References
-
- Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39-55. - PubMed
-
- Bernardi, R., and P. P. Pandolfi. 2003. Role of PML and the PML-nuclear body in the control of programmed cell death. Oncogene 22:9048-9057. - PubMed
-
- Canning, M., C. Boutell, J. Parkinson, and R. D. Everett. 2004. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem. 279:38160-38168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

