Distinct domains of the influenza a virus M2 protein cytoplasmic tail mediate binding to the M1 protein and facilitate infectious virus production
- PMID: 16873274
- PMCID: PMC1563831
- DOI: 10.1128/JVI.00627-06
Distinct domains of the influenza a virus M2 protein cytoplasmic tail mediate binding to the M1 protein and facilitate infectious virus production
Abstract
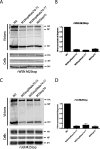
The cytoplasmic tail of the influenza A virus M2 protein is highly conserved among influenza A virus isolates. The cytoplasmic tail appears to be dispensable with respect to the ion channel activity associated with the protein but important for virus morphology and the production of infectious virus particles. Using reverse genetics and transcomplementation assays, we demonstrate that the M2 protein cytoplasmic tail is a crucial mediator of infectious virus production. Truncations of the M2 cytoplasmic tail result in a drastic decrease in infectious virus titers, a reduction in the amount of packaged viral RNA, a decrease in budding events, and a reduction in budding efficiency. The M1 protein binds to the M2 cytoplasmic tail, but the M1 binding site is distinct from the sequences that affect infectious virus particle formation. Influenza A virus strains A/Udorn/72 and A/WSN/33 differ in their requirements for M2 cytoplasmic tail sequences, and this requirement maps to the M1 protein. We conclude that the M2 protein is required for the formation of infectious virus particles, implicating the protein as important for influenza A virus assembly in addition to its well-documented role during virus entry and uncoating.
Figures




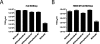
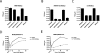


Similar articles
-
Mutations in the Influenza A Virus M1 Protein Enhance Virus Budding To Complement Lethal Mutations in the M2 Cytoplasmic Tail.J Virol. 2017 Dec 14;92(1):e00858-17. doi: 10.1128/JVI.00858-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046451 Free PMC article.
-
The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging.J Virol. 2005 Mar;79(6):3595-605. doi: 10.1128/JVI.79.6.3595-3605.2005. J Virol. 2005. PMID: 15731254 Free PMC article.
-
The influenza virus M2 protein cytoplasmic tail interacts with the M1 protein and influences virus assembly at the site of virus budding.J Virol. 2008 Oct;82(20):10059-70. doi: 10.1128/JVI.01184-08. Epub 2008 Aug 13. J Virol. 2008. PMID: 18701586 Free PMC article.
-
Influenza virus assembly and budding.Virology. 2011 Mar 15;411(2):229-36. doi: 10.1016/j.virol.2010.12.003. Epub 2011 Jan 14. Virology. 2011. PMID: 21237476 Free PMC article. Review.
-
Influenza A Virus M2 Protein: Roles from Ingress to Egress.Int J Mol Sci. 2017 Dec 7;18(12):2649. doi: 10.3390/ijms18122649. Int J Mol Sci. 2017. PMID: 29215568 Free PMC article. Review.
Cited by
-
Structural Analysis of the Roles of Influenza A Virus Membrane-Associated Proteins in Assembly and Morphology.J Virol. 2015 Sep;89(17):8957-66. doi: 10.1128/JVI.00592-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085153 Free PMC article.
-
Mammalian expression of virus-like particles for advanced mimicry of authentic influenza virus.PLoS One. 2010 Mar 22;5(3):e9784. doi: 10.1371/journal.pone.0009784. PLoS One. 2010. PMID: 20339535 Free PMC article.
-
Influenza A Virus M2 Protein Apical Targeting Is Required for Efficient Virus Replication.J Virol. 2018 Oct 29;92(22):e01425-18. doi: 10.1128/JVI.01425-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30158290 Free PMC article.
-
The minimalist architectures of viroporins and their therapeutic implications.Biochim Biophys Acta. 2014 Apr;1838(4):1058-67. doi: 10.1016/j.bbamem.2013.09.004. Epub 2013 Sep 18. Biochim Biophys Acta. 2014. PMID: 24055819 Free PMC article. Review.
-
The influenza C virus CM2 protein can alter intracellular pH, and its transmembrane domain can substitute for that of the influenza A virus M2 protein and support infectious virus production.J Virol. 2012 Jan;86(2):1277-81. doi: 10.1128/JVI.05681-11. Epub 2011 Sep 14. J Virol. 2012. PMID: 21917958 Free PMC article.
References
-
- Barman, S., A. Ali, E. K. Hui, L. Adhikary, and D. P. Nayak. 2001. Transport of viral proteins to the apical membranes and interaction of matrix protein with glycoproteins in the assembly of influenza viruses. Virus Res. 77:61-69. - PubMed
-
- Bourmakina, S. V., and A. Garcia-Sastre. 2003. Reverse genetics studies on the filamentous morphology of influenza A virus. J. Gen. Virol. 84:517-527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

