Hydrophobic alpha-helices 1 and 2 of herpes simplex virus gH interact with lipids, and their mimetic peptides enhance virus infection and fusion
- PMID: 16873275
- PMCID: PMC1563806
- DOI: 10.1128/JVI.00504-06
Hydrophobic alpha-helices 1 and 2 of herpes simplex virus gH interact with lipids, and their mimetic peptides enhance virus infection and fusion
Erratum in
- J Virol. 2007 Mar;81(5):2542
Abstract
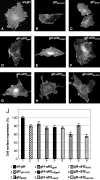
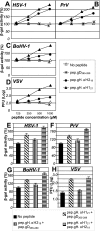
Entry of herpes simplex virus into cells occurs by fusion and requires four glycoproteins. gD serves as the receptor binding glycoprotein. Of the remaining glycoproteins, gH carries structural and functional elements typical of class 1 fusion glycoproteins, in particular alpha-helix 1 (alpha-H1), with properties of a candidate fusion peptide, and two heptad repeats. Here, we characterized alpha-H2 and compared it to alpha-H1. alpha-H2 (amino acids 513 to 531) is of lower hydrophobicity than alpha-H1. Its deletion or mutation decreased virus infection and cell fusion. Its replacement with heterologous fusion peptides did not rescue infection and cell fusion beyond the levels exhibited by the alpha-H2-deleted gH. This contrasts with alpha-H1, which cannot be deleted and can be functionally replaced with heterologous fusion peptides (T. Gianni et al., J. Virol. 79:2931-2940, 2005). Synthetic peptides mimicking alpha-H1 and alpha-H2 induced fusion of nude lipid vesicles. Importantly, they increased infection of herpes simplex virus, pseudorabies virus, bovine herpesvirus 1, and vesicular stomatitis virus. The alpha-H1 mimetic peptide was more effective than the alpha-H2 peptide. Consistent with the findings that gH carries membrane-interacting segments, a soluble form of gH, but not of gD or gB, partitioned with lipid vesicles. Current findings highlight that alpha-H2 is an important albeit nonessential region for virus entry and fusion. alpha-H1 and alpha-H2 share the ability to target the membrane lipids; they contribute to virus entry and fusion, possibly by destabilizing the membranes. However, alpha-H2 differs from alpha-H1 in that it is of lower hydrophobicity and cannot be replaced with heterologous fusion peptides.
Figures





Similar articles
-
A heptad repeat in herpes simplex virus 1 gH, located downstream of the alpha-helix with attributes of a fusion peptide, is critical for virus entry and fusion.J Virol. 2005 Jun;79(11):7042-9. doi: 10.1128/JVI.79.11.7042-7049.2005. J Virol. 2005. PMID: 15890943 Free PMC article.
-
Heptad repeat 2 in herpes simplex virus 1 gH interacts with heptad repeat 1 and is critical for virus entry and fusion.J Virol. 2006 Mar;80(5):2216-24. doi: 10.1128/JVI.80.5.2216-2224.2006. J Virol. 2006. PMID: 16474129 Free PMC article.
-
The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the herpesviridae family.J Virol. 2005 Mar;79(5):2931-40. doi: 10.1128/JVI.79.5.2931-2940.2005. J Virol. 2005. PMID: 15709012 Free PMC article.
-
Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry.Virology. 2006 Jan 5;344(1):17-24. doi: 10.1016/j.virol.2005.09.016. Virology. 2006. PMID: 16364731 Review.
-
The multipartite system that mediates entry of herpes simplex virus into the cell.Rev Med Virol. 2007 Sep-Oct;17(5):313-26. doi: 10.1002/rmv.546. Rev Med Virol. 2007. PMID: 17573668 Review.
Cited by
-
Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies.J Virol. 2007 Oct;81(20):11468-78. doi: 10.1128/JVI.01364-07. Epub 2007 Aug 8. J Virol. 2007. PMID: 17686835 Free PMC article.
-
Reevaluating herpes simplex virus hemifusion.J Virol. 2010 Nov;84(22):11814-21. doi: 10.1128/JVI.01615-10. Epub 2010 Sep 15. J Virol. 2010. PMID: 20844038 Free PMC article.
-
Peptides containing membrane-interacting motifs inhibit herpes simplex virus type 1 infectivity.Peptides. 2008 Sep;29(9):1461-71. doi: 10.1016/j.peptides.2008.04.022. Epub 2008 May 17. Peptides. 2008. PMID: 18572274 Free PMC article.
-
Herpes simplex virus glycoprotein B associates with target membranes via its fusion loops.J Virol. 2009 Jul;83(13):6825-36. doi: 10.1128/JVI.00301-09. Epub 2009 Apr 15. J Virol. 2009. PMID: 19369321 Free PMC article.
-
Herpes simplex virus glycoproteins H/L bind to cells independently of {alpha}V{beta}3 integrin and inhibit virus entry, and their constitutive expression restricts infection.J Virol. 2010 Apr;84(8):4013-25. doi: 10.1128/JVI.02502-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147400 Free PMC article.
References
-
- Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant, and downmodulates their cell surface expression. J. Virol. 78:8015-8025. - PMC - PubMed
-
- Babic, N., B. G. Klupp, B. Makoschey, A. Karger, A. Flamand, and T. C. Mettenleiter. 1996. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J. Gen. Virol. 77:2277-2285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

