Dynamic clamp: a powerful tool in cardiac electrophysiology
- PMID: 16873403
- PMCID: PMC1890360
- DOI: 10.1113/jphysiol.2006.115840
Dynamic clamp: a powerful tool in cardiac electrophysiology
Abstract
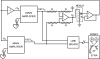
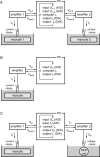
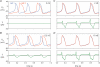
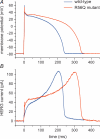
Dynamic clamp is a collection of closely related techniques that have been employed in cardiac electrophysiology to provide direct answers to numerous research questions regarding basic cellular mechanisms of action potential formation, action potential transfer and action potential synchronization in health and disease. Building on traditional current clamp, dynamic clamp was initially used to create virtual gap junctions between isolated myocytes. More recent applications include the embedding of a real pacemaking myocyte in a simulated network of atrial or ventricular cells and the insertion of virtual ion channels, either simulated in real time or simultaneously recorded from an expression system, into the membrane of an isolated myocyte. These applications have proven that dynamic clamp, which is characterized by the real-time evaluation and injection of simulated membrane current, is a powerful tool in cardiac electrophysiology. Here, each of the three different experimental configurations used in cardiac electrophysiology is reviewed. Also, directions are given for the implementation of dynamic clamp in the cardiac electrophysiology laboratory. With the growing interest in the application of dynamic clamp in cardiac electrophysiology, it is anticipated that dynamic clamp will also prove to be a powerful tool in basic research on biological pacemakers and in identification of specific ion channels as targets for drug development.
Figures

Similar articles
-
Dynamic clamp as a tool to study the functional effects of individual membrane currents.Methods Mol Biol. 2014;1183:309-26. doi: 10.1007/978-1-4939-1096-0_20. Methods Mol Biol. 2014. PMID: 25023318
-
Advances in patch clamp technique: towards higher quality and quantity.Gen Physiol Biophys. 2012 Jun;31(2):131-40. doi: 10.4149/gpb_2012_016. Gen Physiol Biophys. 2012. PMID: 22781816 Review.
-
Cardiac channelopathies studied with the dynamic action potential-clamp technique.Methods Mol Biol. 2007;403:233-50. doi: 10.1007/978-1-59745-529-9_16. Methods Mol Biol. 2007. PMID: 18827999
-
The patch clamp technique: principles and technical considerations.J Vet Cardiol. 2007 May;9(1):25-37. doi: 10.1016/j.jvc.2007.02.001. Epub 2007 May 16. J Vet Cardiol. 2007. PMID: 17689466 Review.
-
[Advancement of patch-clamp technique and its application in drug high-throughput screening].Sheng Li Ke Xue Jin Zhan. 2005 Apr;36(2):125-9. Sheng Li Ke Xue Jin Zhan. 2005. PMID: 16222971 Review. Chinese.
Cited by
-
Dynamic action potential clamp predicts functional separation in mild familial and severe de novo forms of SCN2A epilepsy.Proc Natl Acad Sci U S A. 2018 Jun 12;115(24):E5516-E5525. doi: 10.1073/pnas.1800077115. Epub 2018 May 29. Proc Natl Acad Sci U S A. 2018. PMID: 29844171 Free PMC article.
-
Quantification of repolarization reserve to understand interpatient variability in the response to proarrhythmic drugs: a computational analysis.Heart Rhythm. 2011 Nov;8(11):1749-55. doi: 10.1016/j.hrthm.2011.05.023. Epub 2011 Jun 7. Heart Rhythm. 2011. PMID: 21699863 Free PMC article.
-
Applications of Dynamic Clamp to Cardiac Arrhythmia Research: Role in Drug Target Discovery and Safety Pharmacology Testing.Front Physiol. 2018 Jan 4;8:1099. doi: 10.3389/fphys.2017.01099. eCollection 2017. Front Physiol. 2018. PMID: 29354069 Free PMC article. Review.
-
Injection of IK1 through dynamic clamp can make all the difference in patch-clamp studies on hiPSC-derived cardiomyocytes.Front Physiol. 2023 Dec 12;14:1326160. doi: 10.3389/fphys.2023.1326160. eCollection 2023. Front Physiol. 2023. PMID: 38152247 Free PMC article.
-
Simultaneous recording of electrical activity and the underlying ionic currents in NG108-15 cells cultured on gold substrate.Heliyon. 2018 Mar 1;4(2):e00550. doi: 10.1016/j.heliyon.2018.e00550. eCollection 2018 Feb. Heliyon. 2018. PMID: 29560462 Free PMC article.
References
-
- Barabanov M, Yodaiken V. Introducing real-time Linux. Linux J. 1997;34:19–23.
-
- Butera RJ, Jr, Wilson CG, Delnegro CA, Smith JC. A methodology for achieving high-speed rates for artificial conductance injection in electrically excitable biological cells. IEEE Trans Biomed Eng. 2001;48:1460–1470. - PubMed
-
- Christini DJ, Stein KM, Markowitz SM, Lerman BB. Practical real-time computing system for biomedical experiment interface. Ann Biomed Eng. 1999;27:180–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

