A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation
- PMID: 16873404
- PMCID: PMC1890358
- DOI: 10.1113/jphysiol.2006.115766
A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation
Abstract
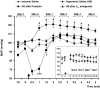
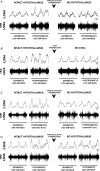
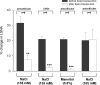
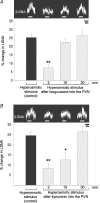
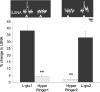
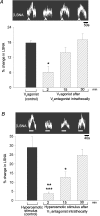
An elevation in plasma osmolality elicits a complex neurohumoral response, including an activation of the sympathetic nervous system and an increase in arterial pressure. Using a combination of in vivo and in situ rat preparations, we sought to investigate whether hypothalamic vasopressinergic spinally projecting neurones are activated during increases in plasma osmolality to elicit sympathoexcitation. Hypertonic saline (HS, i.v. bolus), which produced a physiological increase in plasma osmolality to 299 +/- 1 mosmol (kg water)(-1), elicited an immediate increase in mean arterial pressure (MAP) (from 101 +/- 1 to 121 +/- 3 mmHg) in vivo. Pre-treatment with prazosin reversed the HS-induced pressor response to a hypotensive response (from 121 +/- 3 to 68 +/- 2 mmHg), indicating significant activation of the sympathetic nervous system. In an in situ arterially perfused decorticate rat preparation, hyperosmotic perfusate consisted of either 135 mm NaCl, or a non-NaCl osmolyte, mannitol (0.5%); both increased lumbar sympathetic nerve activity (LSNA) by 32 +/- 5% (NaCl) and 21 +/- 1% (mannitol), which was attenuated after precollicular transection (7 +/- 3% and 1 +/- 1%, respectively). Remaining experiments used the NaCl hyperosmotic stimulus. In separate preparations the hyperosmotic-induced sympathoexcitation (21 +/- 2%) was also significantly attenuated after transection of the circumventricular organs (2 +/- 1%). Either isoguvacine (a GABA(A) receptor agonist) or kynurenic acid (a non-selective ionotropic glutamate receptor antagonist) microinjected bilaterally into the paraventricular nucleus (PVN) attenuated the increase in LSNA induced by the hyperosmotic stimulus (control: 25 +/- 2%; after isoguvacine: 7 +/- 2%; after kynurenic: 8 +/- 3%). Intrathecal injection of a V(1a) receptor antagonist also reduced the increase in LSNA elicited by the hyperosmotic stimulus (control: 29 +/- 6%; after blocker: 4 +/- 1%). These results suggest that a physiological hyperosmotic stimulus produces sympathetically mediated hypertension in conscious rats. These data are substantiated by the in situ decorticate preparation in which sympathoexcitation was also evoked by comparable hyperosmotic stimulation. Our findings demonstrate the importance of vasopressin acting on spinal V(1a) receptors for mediating sympathoexcitatory response to acute salt loading.
Figures

References
-
- Akins VF, Bealer SL. Brain histamine regulates pressor responses to peripheral hyperosmolality. Am J Physiol. 1990;259:R507–R513. - PubMed
-
- Antunes VR, Yao ST, Murphy D, Pickering AE, Paton JFR. An in situ preparation for studying hypothalamic control of sympathetic activity. J Physiol. 2005;567P:PC51.
-
- Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84:169–208. - PubMed
-
- Badoer E. Cardiovascular role of parvocellular neurones in the paraventricular nucleus of the hypothalamus. News Physiol Sci. 1996;11:43–47.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

