Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle
- PMID: 16873412
- PMCID: PMC1890364
- DOI: 10.1113/jphysiol.2006.113175
Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle
Abstract
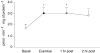
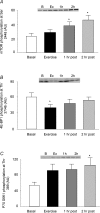
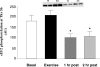
Resistance exercise is a potent stimulator of muscle protein synthesis and muscle cell growth, with the increase in protein synthesis being detected within 2-3 h post-exercise and remaining elevated for up to 48 h. However, during exercise, muscle protein synthesis is inhibited. An increase in AMP-activated protein kinase (AMPK) activity has recently been shown to decrease mammalian target of rapamycin (mTOR) signalling to key regulators of translation initiation. We hypothesized that the cellular mechanism for the inhibition of muscle protein synthesis during an acute bout of resistance exercise in humans would be associated with an activation of AMPK and an inhibition of downstream components of the mTOR pathway (4E-BP1 and S6K1). We studied 11 subjects (seven men, four women) before, during, and for 2 h following a bout of resistance exercise. Muscle biopsy specimens were collected at each time point from the vastus lateralis. We utilized immunoprecipitation and immunoblotting methods to measure muscle AMPKalpha2 activity, and mTOR-associated upstream and downstream signalling proteins, and stable isotope techniques to measure muscle fractional protein synthetic rate (FSR). AMPKalpha2 activity (pmol min(-1) (mg protein)(-1)) at baseline was 1.7 +/- 0.3, increased immediately post-exercise (3.0 +/- 0.6), and remained elevated at 1 h post-exercise (P < 0.05). Muscle FSR decreased during exercise and was significantly increased at 1 and 2 h post-exercise (P < 0.05). Phosphorylation of 4E-BP1 at Thr37/46 was significantly reduced immediately post-exercise (P < 0.05). We conclude that AMPK activation and a reduced phosphorylation of 4E-BP1 may contribute to the inhibition of muscle protein synthesis during resistance exercise. However, by 1-2 h post-exercise, muscle protein synthesis increased in association with an activation of protein kinase B, mTOR, S6K1 and eEF2.
Figures


References
-
- Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–E1101. - PubMed
-
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. - PubMed
-
- Baar K, Esser K. Phosphorylation of p70 (S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–C127. - PubMed
-
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268:E514–E520. - PubMed
-
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

