Activation of estrogen receptor-alpha reduces aortic smooth muscle differentiation
- PMID: 16873715
- PMCID: PMC1905928
- DOI: 10.1161/01.RES.0000238376.72592.a2
Activation of estrogen receptor-alpha reduces aortic smooth muscle differentiation
Abstract
Women are at high risk of dying from unrecognized cardiovascular disease. Many differences in cardiovascular disease between men and women appear to be mediated by vascular smooth muscle cells (SMC). Because estrogen reduces the proliferation of SMC, we hypothesized that activation of estrogen receptor-alpha (ERalpha) by agonists or by growth factors altered SMC function. To determine the effect of growth factors, estrogen, and ERalpha expression on SMC differentiation, human aortic SMC were cultured in serum-free conditions for 10 days. SMC from men had lower spontaneous expression of ERalpha and higher levels of the differentiation markers calponin and smooth muscle alpha-actin than SMC from women. When SMC containing low expression of ERalpha were transduced with a lentivirus containing ERalpha, activation of the receptor by ligands or growth factors reduced differentiation markers. Conversely, inhibiting ERalpha expression by small interfering RNA (siRNA) in cells expressing high levels of ERalpha enhanced the expression of differentiation markers. ERalpha expression and activation reduced the phosphorylation of Smad2, a signaling molecule important in differentiation of SMC and initiated cell death through cleavage of caspase-3. We conclude that ERalpha activation switched SMC to a dedifferentiated phenotype and may contribute to plaque instability.
Conflict of interest statement
Figures


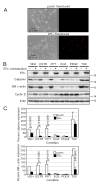
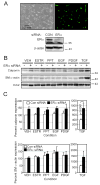
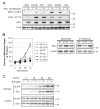

Comment in
-
Estrogen and different aspects of vascular disease in women and men.Circ Res. 2006 Sep 1;99(5):459-61. doi: 10.1161/01.RES.0000241056.84659.59. Circ Res. 2006. PMID: 16946140 Review. No abstract available.
References
-
- American Heart Association . Heart Disease and Stroke Statistics - 2005 Update. Dallas, Texas: 2005.
-
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
-
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. - PubMed
-
- Grodstein F, Manson JE, Stampfer MJ. Hormone Therapy and Coronary Heart Disease: The Role of Time since Menopause and Age at Hormone Initiation. J Womens Health (Larchmt ) 2006;15:35–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

