Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes
- PMID: 16874021
- PMCID: PMC1584280
- DOI: 10.4161/auto.1.1.1589
Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes
Abstract
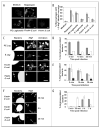
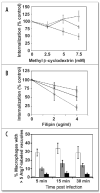
Macrophages activate autophagy as an immediate response to Legionella pneumophila infection, but what marks the pathogen phagosome as a target for the autophagy machinery is not known. Because a variety of bacteria, parasites, viruses, and toxins that associate with the endoplasmic reticulum enter host cells by a cholesterol-dependent route, we tested the hypothesis that autophagy is triggered when microbes engage components of lipid raft domains. As the intracellular respiratory pathogen L. pneumophila or the extracellular uropathogen FimH(+) Escherichia coli entered macrophages by a cholesterol-sensitive mechanism, they immediatezly resided in vacuoles rich in glycosylphosphatidylinositol moieties and the autophagy enzyme Atg7. As expected for autophagosomes, the vacuoles sequentially acquired the endoplasmic reticulum protein BiP, the autophagy markers Atg8 and monodansyl-cadaverine, and the lysosomal protein LAMP-1. A robust macrophage response to the pathogens was cholesterol-dependent, since fewer Atg7-rich vacuoles were observed when macrophages were pretreated with methyl-beta-cyclodextrin or filipin. A model in which macrophages exploit autophagy to capture pathogens within the lipid raft pathway for antigen presentation prior to disposal in lysosomes is discussed.
Figures


References
-
- Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, Abraham SN. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389:636–9. - PubMed
-
- Gatfield J, Pieters J. Essential role for cholesterol in entry of Mycobacteria into macrophages. Science. 2000;288:1647–50. - PubMed
-
- Manes S, del Real G, Martinez AC. Pathogens: Raft hijackers. Nat Rev Immunol. 2003;3:557–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
