Cellular and matrix contributions to tissue construct stiffness increase with cellular concentration
- PMID: 16874557
- PMCID: PMC3689290
- DOI: 10.1007/s10439-006-9160-2
Cellular and matrix contributions to tissue construct stiffness increase with cellular concentration
Erratum in
- Ann Biomed Eng. 2007 Feb;35(2):320
-
Cellular and Matrix Contributions to Tissue Construct Stiffness Increase with Cellular Concentration.Ann Biomed Eng. 2007 Feb;35(2):320. doi: 10.1007/s10439-006-9215-4. Ann Biomed Eng. 2007. PMID: 27520028 No abstract available.
Abstract
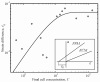
The mechanics of bio-artificial tissue constructs result from active and passive contributions of cells and extracellular matrix (ECM). We delineated these for a fibroblast-populated matrix (FPM) consisting of chick embryo fibroblast cells in a type I collagen ECM through mechanical testing, mechanical modeling, and selective biochemical elimination of tissue components. From a series of relaxation tests, we found that contributions to overall tissue mechanics from both cells and ECM increase exponentially with the cell concentration. The force responses in these relaxation tests exhibited a logarithmic decay over the 3600 second test duration. The amplitudes of these responses were nearly linear with the amplitude of the applied stretch. The active component of cellular forces rose dramatically for FPMs containing higher cell concentrations.
Figures











Similar articles
-
Cell mechanics studied by a reconstituted model tissue.Biophys J. 2000 Nov;79(5):2353-68. doi: 10.1016/S0006-3495(00)76481-2. Biophys J. 2000. PMID: 11053115 Free PMC article.
-
Cellular and matrix mechanics of bioartificial tissues during continuous cyclic stretch.Ann Biomed Eng. 2006 Nov;34(11):1678-90. doi: 10.1007/s10439-006-9153-1. Epub 2006 Oct 11. Ann Biomed Eng. 2006. PMID: 17033741 Free PMC article.
-
One-dimensional viscoelastic behavior of fibroblast populated collagen matrices.J Biomech Eng. 2003 Oct;125(5):719-25. doi: 10.1115/1.1614818. J Biomech Eng. 2003. PMID: 14618931
-
Whole cell mechanics of contractile fibroblasts: relations between effective cellular and extracellular matrix moduli.Philos Trans A Math Phys Eng Sci. 2010 Feb 13;368(1912):635-54. doi: 10.1098/rsta.2009.0240. Philos Trans A Math Phys Eng Sci. 2010. PMID: 20047943 Free PMC article. Review.
-
Assessing the functional mechanical properties of bioengineered organs with emphasis on the lung.J Cell Physiol. 2014 Sep;229(9):1134-40. doi: 10.1002/jcp.24600. J Cell Physiol. 2014. PMID: 24604423 Review.
Cited by
-
Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression.Tissue Eng Part A. 2011 Apr;17(7-8):1039-53. doi: 10.1089/ten.TEA.2009.0499. Epub 2011 Jan 9. Tissue Eng Part A. 2011. PMID: 21091338 Free PMC article.
-
Correction of bias in the estimation of cell volume fraction from histology sections.J Biomech. 2020 May 7;104:109705. doi: 10.1016/j.jbiomech.2020.109705. Epub 2020 Mar 2. J Biomech. 2020. PMID: 32247525 Free PMC article.
-
Stress amplification during development of the tendon-to-bone attachment.Biomech Model Mechanobiol. 2014 Oct;13(5):973-83. doi: 10.1007/s10237-013-0548-2. Epub 2013 Dec 27. Biomech Model Mechanobiol. 2014. PMID: 24370852 Free PMC article.
-
Fibrotic microtissue array to predict anti-fibrosis drug efficacy.Nat Commun. 2018 May 25;9(1):2066. doi: 10.1038/s41467-018-04336-z. Nat Commun. 2018. PMID: 29802256 Free PMC article.
-
Swelling of Collagen-Hyaluronic Acid Co-Gels: An In Vitro Residual Stress Model.Ann Biomed Eng. 2016 Oct;44(10):2984-2993. doi: 10.1007/s10439-016-1636-0. Epub 2016 May 5. Ann Biomed Eng. 2016. PMID: 27150674 Free PMC article.
References
-
- Bellows CG, Melcher AH, Aubin JE. Cells of different types compress and remodel collagen to markedly different degrees. Journal of Cell Science. 1981;50:299–314. - PubMed
-
- Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25(17):3699–3706. - PubMed
-
- Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair. Regen. 2005;13(1):7–12. - PubMed
-
- Eshelby JD. The determination of the elastic field outside an ellipsoidal inclusion and related problems. Proc. Roy. Soc. A. 1957:241.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

