Fate of endogenous stem/progenitor cells following spinal cord injury
- PMID: 16874803
- PMCID: PMC2553041
- DOI: 10.1002/cne.21065
Fate of endogenous stem/progenitor cells following spinal cord injury
Abstract
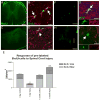
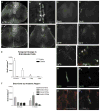
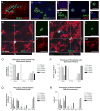
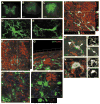
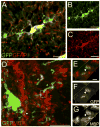
The adult mammalian spinal cord contains neural stem and/or progenitor cells that slowly multiply throughout life and differentiate exclusively into glia. The contribution of adult progenitors to repair has been highlighted in recent studies, demonstrating extensive cell proliferation and gliogenesis following central nervous system (CNS) trauma. The present experiments aimed to determine the relative roles of endogenously dividing progenitor cells versus quiescent progenitor cells in posttraumatic gliogenesis. Using the mitotic indicator bromodeoxyuridine (BrdU) and a retroviral vector, we found that, in the adult female Fisher 344 rat, endogenously dividing neural progenitors are acutely vulnerable in response to T8 dorsal hemisection spinal cord injury. We then studied the population of cells that divide postinjury in the injury epicenter by delivering BrdU or retrovirus at 24 hours after spinal cord injury. Animals were euthanized at five timepoints postinjury, ranging from 6 hours to 9 weeks after BrdU delivery. At all timepoints, we observed extensive proliferation of ependymal and periependymal cells that immunohistochemically resembled stem/progenitor cells. BrdU+ incorporation was noted to be prominent in NG2-immunoreactive progenitors that matured into oligodendrocytes, and in a transient population of microglia. Using a green fluorescence protein (GFP) hematopoietic chimeric mouse, we determined that 90% of the dividing cells in this early proliferation event originate from the spinal cord, whereas only 10% originate from the bone marrow. Our results suggest that dividing, NG2-expressing progenitor cells are vulnerable to injury, but a separate, immature population of neural stem and/or progenitor cells is activated by injury and rapidly divides to replace this vulnerable population.
Copyright (c) 2006 Wiley-Liss, Inc.
Figures
References
-
- Adrian EK, Jr, Walker BE. Incorporation of thymidine-H3 by cells in normal and injured mouse spinal cord. J Neuropathol Exp Neurol. 1962;21:597– 609. - PubMed
-
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. - PubMed
-
- Berry M, Hubbard P, Butt AM. Cytology and lineage of NG2-positive glia. J Neurocytol. 2002;31:457– 467. - PubMed
-
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. - PubMed
-
- Chen ZJ, Negra M, Levine A, Ughrin Y, Levine JM. Oligodendrocyte precursor cells: reactive cells that inhibit axon growth and regeneration. J Neurocytol. 2002;31:481– 495. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

