c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis
- PMID: 16875436
- PMCID: PMC1526764
- DOI: 10.1371/journal.pbio.0040268
c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis
Abstract
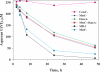
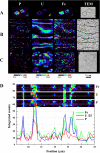
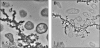
Modern approaches for bioremediation of radionuclide contaminated environments are based on the ability of microorganisms to effectively catalyze changes in the oxidation states of metals that in turn influence their solubility. Although microbial metal reduction has been identified as an effective means for immobilizing highly-soluble uranium(VI) complexes in situ, the biomolecular mechanisms of U(VI) reduction are not well understood. Here, we show that c-type cytochromes of a dissimilatory metal-reducing bacterium, Shewanella oneidensis MR-1, are essential for the reduction of U(VI) and formation of extracellular UO(2) nanoparticles. In particular, the outer membrane (OM) decaheme cytochrome MtrC (metal reduction), previously implicated in Mn(IV) and Fe(III) reduction, directly transferred electrons to U(VI). Additionally, deletions of mtrC and/or omcA significantly affected the in vivo U(VI) reduction rate relative to wild-type MR-1. Similar to the wild-type, the mutants accumulated UO(2) nanoparticles extracellularly to high densities in association with an extracellular polymeric substance (EPS). In wild-type cells, this UO(2)-EPS matrix exhibited glycocalyx-like properties and contained multiple elements of the OM, polysaccharide, and heme-containing proteins. Using a novel combination of methods including synchrotron-based X-ray fluorescence microscopy and high-resolution immune-electron microscopy, we demonstrate a close association of the extracellular UO(2) nanoparticles with MtrC and OmcA (outer membrane cytochrome). This is the first study to our knowledge to directly localize the OM-associated cytochromes with EPS, which contains biogenic UO(2) nanoparticles. In the environment, such association of UO(2) nanoparticles with biopolymers may exert a strong influence on subsequent behavior including susceptibility to oxidation by O(2) or transport in soils and sediments.
Figures



Comment in
-
Cultivating bacteria's taste for toxic waste.PLoS Biol. 2006 Aug;4(8):e282. doi: 10.1371/journal.pbio.0040282. Epub 2006 Aug 8. PLoS Biol. 2006. PMID: 20076626 Free PMC article. No abstract available.
References
-
- Lovley DR, Holmes DE, Nevin KP. Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol. 2004;49:219–286. - PubMed
-
- Nealson KH, Saffarini D. Iron and manganese in anaerobic respiration: Environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. - PubMed
-
- Rusin PA, Quintana L, Brainard JR, Strietelmeier BA, Tait CD, et al. Solubilization of plutonium hydrous oxide by iron-reducing bacteria. Environ Sci Technol. 1994;28:1686–1690. - PubMed
-
- Liu C, Gorby YA, Zachara JM, Fredrickson JK, Brown CF. Reduction kinetics of Fe(III), Co(III), U(VI), Cr(VI), and Tc(VII) in cultures of dissimilatory metal-reducing bacteria. Biotechnol Bioeng. 2002;80:637–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

