Heat shock protein 27: its potential role in vascular disease
- PMID: 16875491
- PMCID: PMC2517372
- DOI: 10.1111/j.1365-2613.2006.00484.x
Heat shock protein 27: its potential role in vascular disease
Abstract
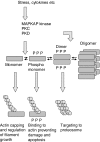
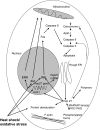
Heat shock proteins are molecular chaperones that have an ability to protect proteins from damage induced by environmental factors such as free radicals, heat, ischaemia and toxins, allowing denatured proteins to adopt their native configuration. Heat shock protein-27 (Hsp27) is a member of the small Hsp (sHsp) family of proteins, and has a molecular weight of approximately 27 KDa. In addition to its role as a chaperone, it has also been reported to have many additional functions. These include effects on the apoptotic pathway, cell movement and embryogenesis. In this review, we have focused on its possible role in vascular disease.
Figures

References
-
- Abu Shama KM, Suzuki A, Harada K, et al. Transient up-regulation of myotonic dystrophy protein kinase- binding protein, MKBP, and HSP27 in the neonatal myocardium. Cell. Struct. Funct. 1999;24:1–4. - PubMed
-
- Afek A, George J, Gilburd B, et al. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J. Autoimmun. 2000;14:115–121. - PubMed
-
- Akamatsu S, Nakajima K, Ishisaki A, et al. Vasopressin phosphorylates HSP27 in aortic smooth muscle cells. J. Cell. Biochem. 2004;92:1203–1211. - PubMed
-
- Arata S, Hamaguchi S, Nose K. Effects of the overexpression of the small heat-shock protein, Hsp27, on the sensitivity of human fibroblast cells exposed to oxidative stress. J. Cell. Physiol. 1995;163:458–465. - PubMed
-
- Arrigo AP. sHsp as novel regulators of programmed cell death and tumorigenicity. Pathol. Biol. (Pari.) 2000;48:280–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

