Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication
- PMID: 16875760
- PMCID: PMC7119042
- DOI: 10.1016/j.ejmech.2006.03.024
Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication
Abstract
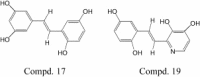
Stilbene derivatives have wide range of activities. In an effort to find other potential activities of this kind of compounds, 17 derivatives, including resveratrol, were synthesized. Twelve of them were evaluated for their antiviral potential against severe acute respiratory syndrome (SARS)-CoV-induced cytopathicity in Vero E6 cell culture. The result showed that SARS virus was totally inhibited by compounds 17 and 19 (<or=0.5 mg ml(-1)) and no significant cytotoxic effects were observed in vitro.
Figures
Similar articles
-
Antiviral activity of nucleoside analogues against SARS-coronavirus (SARS-coV).Antivir Chem Chemother. 2006;17(5):285-9. doi: 10.1177/095632020601700506. Antivir Chem Chemother. 2006. PMID: 17176633
-
Inhibitory effect of mizoribine and ribavirin on the replication of severe acute respiratory syndrome (SARS)-associated coronavirus.Antiviral Res. 2005 Jun;66(2-3):159-63. doi: 10.1016/j.antiviral.2005.01.003. Epub 2005 Feb 19. Antiviral Res. 2005. PMID: 15911031 Free PMC article.
-
The antiviral effect of interferon-beta against SARS-coronavirus is not mediated by MxA protein.J Clin Virol. 2004 Jul;30(3):211-3. doi: 10.1016/j.jcv.2003.11.013. J Clin Virol. 2004. PMID: 15135736 Free PMC article.
-
Interferon-beta 1a and SARS coronavirus replication.Emerg Infect Dis. 2004 Feb;10(2):317-9. doi: 10.3201/eid1002.030482. Emerg Infect Dis. 2004. PMID: 15030704 Free PMC article.
-
Synthesis of novel test compounds for antiviral chemotherapy of severe acute respiratory syndrome (SARS).Curr Med Chem. 2005;12(18):2095-162. doi: 10.2174/0929867054637644. Curr Med Chem. 2005. PMID: 16101496 Review.
Cited by
-
More Than Resveratrol: New Insights into Stilbene-Based Compounds.Biomolecules. 2020 Jul 27;10(8):1111. doi: 10.3390/biom10081111. Biomolecules. 2020. PMID: 32726968 Free PMC article. Review.
-
Anticoronavirus and Immunomodulatory Phenolic Compounds: Opportunities and Pharmacotherapeutic Perspectives.Biomolecules. 2021 Aug 23;11(8):1254. doi: 10.3390/biom11081254. Biomolecules. 2021. PMID: 34439920 Free PMC article. Review.
-
Synthesis, modification and docking studies of 5-sulfonyl isatin derivatives as SARS-CoV 3C-like protease inhibitors.Bioorg Med Chem. 2014 Jan 1;22(1):292-302. doi: 10.1016/j.bmc.2013.11.028. Epub 2013 Nov 21. Bioorg Med Chem. 2014. PMID: 24316352 Free PMC article.
-
Phytopharmaceuticals mediated Furin and TMPRSS2 receptor blocking: can it be a potential therapeutic option for Covid-19?Phytomedicine. 2021 May;85:153396. doi: 10.1016/j.phymed.2020.153396. Epub 2020 Oct 28. Phytomedicine. 2021. PMID: 33380375 Free PMC article. Review.
-
Resveratrol attenuates cortical neuron activity: roles of large conductance calcium-activated potassium channels and voltage-gated sodium channels.J Biomed Sci. 2016 May 21;23(1):47. doi: 10.1186/s12929-016-0259-y. J Biomed Sci. 2016. PMID: 27209372 Free PMC article.
References
-
- Wieslaw P., Bogdan K. IL Farmaco. 1999;54:584–587. - PubMed
-
- Ali M.A., Kondo K., Tsuda Y. Chem. Pharm. Bull. (Tokyo) 1992;40:1130–1136.
-
- Boonlaksiri C., Oonanant W., Kongsaeree P., Kittakoop P., Tanticharoen M., Thebtaranonth Y. Phytochem. 2000;54:415–417. - PubMed
-
- Cai Y.J., Fang J.G., Ma L.P., Yang L., Liu Z.L. Biochim. Biophys. Acta. 2003;1637:31–38. - PubMed
-
- Matsuda H., Morikawa T., Toguchida I., Park J.Y., Harima S., Yoshikawa M. Bioorg. Med. Chem. 2001;9:41–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

