Purification and characterization of a thermostable, haloalkaliphilic extracellular serine protease from the extreme halophilic archaeon Halogeometricum borinquense strain TSS101
- PMID: 16877321
- PMCID: PMC2685586
- DOI: 10.1155/2006/430763
Purification and characterization of a thermostable, haloalkaliphilic extracellular serine protease from the extreme halophilic archaeon Halogeometricum borinquense strain TSS101
Abstract
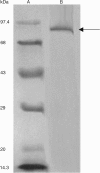
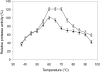
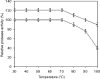
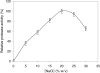
A novel haloalkaliphilic, thermostable serine protease was purified from the extreme halophilic archaeon, Halogeometricum borinquense strain TSS101. The protease was isolated from a stationary phase culture, purified 116-fold with 18% yield and characterized biochemically. The molecular mass of the purified enzyme was estimated to be 86 kDa. The enzyme showed the highest activity at 60 degrees C and pH 10.0 in 20% NaCl. The enzyme had high activity over the pH range from 6.0 to 10.0. Enzymatic activity was strongly inhibited by 1 mM phenyl methylsulfonyl fluoride, but activity was increased 59% by 0.1% cetyltrimethylammonium bromide. The enzyme exhibited relatively high thermal stability, retaining 80% of its activity after 1 h at 90 degrees C. Thermostability increased in the presence of Ca2+. The stability of the enzyme was maintained in 10% sucrose and in the absence of NaCl.
Figures


Similar articles
-
Optimization of culture conditions for the production of haloalkaliphilic thermostable protease from an extremely halophilic archaeon Halogeometricum sp. TSS101.Lett Appl Microbiol. 2006 Oct;43(4):385-91. doi: 10.1111/j.1472-765X.2006.01980.x. Lett Appl Microbiol. 2006. PMID: 16965368
-
Purification and stability characteristics of an alkaline serine protease from a newly isolated Haloalkaliphilic bacterium sp. AH-6.J Ind Microbiol Biotechnol. 2008 Feb;35(2):121-31. doi: 10.1007/s10295-007-0273-x. Epub 2007 Nov 10. J Ind Microbiol Biotechnol. 2008. PMID: 17994257
-
Extracellular protease of Natrialba magadii: purification and biochemical characterization.Extremophiles. 2000 Jun;4(3):181-8. doi: 10.1007/s007920070033. Extremophiles. 2000. PMID: 10879563
-
[Purification and characterization of extracellular halophilic protease from haloarchaea Natrinema sp. R6-5].Wei Sheng Wu Xue Bao. 2007 Feb;47(1):161-3. Wei Sheng Wu Xue Bao. 2007. PMID: 17436645 Chinese.
-
Purification and biochemical characterization of the haloalkaliphilic archaeon Natronococcus occultus extracellular serine protease.J Basic Microbiol. 2001;41(6):375-83. doi: 10.1002/1521-4028(200112)41:6<375::AID-JOBM375>3.0.CO;2-0. J Basic Microbiol. 2001. PMID: 11802548
Cited by
-
Nature and bioprospecting of haloalkaliphilics: a review.World J Microbiol Biotechnol. 2020 Apr 23;36(5):66. doi: 10.1007/s11274-020-02841-2. World J Microbiol Biotechnol. 2020. PMID: 32323057 Review.
-
Hly176B, a low-salt tolerant halolysin from the haloarchaeon Haloarchaeobius sp. FL176.World J Microbiol Biotechnol. 2023 May 9;39(7):189. doi: 10.1007/s11274-023-03632-1. World J Microbiol Biotechnol. 2023. PMID: 37157004
-
Potential for industrial products from the halophilic Archaea.J Ind Microbiol Biotechnol. 2011 Oct;38(10):1635-47. doi: 10.1007/s10295-011-1021-9. Epub 2011 Aug 19. J Ind Microbiol Biotechnol. 2011. PMID: 21853327 Review.
-
Isolation, characterization and exploring biotechnological potential of halophilic archaea from salterns of western India.3 Biotech. 2018 Jan;8(1):45. doi: 10.1007/s13205-017-1072-3. Epub 2018 Jan 3. 3 Biotech. 2018. PMID: 29354356 Free PMC article.
-
A novel halolysin without C-terminal extension from an extremely halophilic archaeon.Appl Microbiol Biotechnol. 2022 Apr;106(8):3009-3019. doi: 10.1007/s00253-022-11903-4. Epub 2022 Apr 18. Appl Microbiol Biotechnol. 2022. PMID: 35435453
References
-
- Adams M.W.W., Kelly R.M. Enzymes from microorganisms in extreme environments. Chem. Eng. News. 1995;73:32–42.
-
- Bhosale S.H., Rao M.B., Deshpande V.V., Srinivasan M.C. Thermostability of high activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20). Enzyme Microb. Technol. 1995;17:136–139.
-
- Cowan D.A., Daniel R.M. Purification and some properties of an extracellular protease (caldolysin) from an extreme thermophile. Biochim. Biophys. Acta. 1982;705:293–305. - PubMed
-
- Da Costa M.S., Santos H., Galinski E.A. An overview of the role and diversity of compatible solutes. Adv. Biochem. Eng. Biotechnol. 1998;61:117–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

