Hematopoietic stem cells: the paradigmatic tissue-specific stem cell
- PMID: 16877336
- PMCID: PMC1698791
- DOI: 10.2353/ajpath.2006.060312
Hematopoietic stem cells: the paradigmatic tissue-specific stem cell
Erratum in
- Am J Pathol. 2006 Nov;169(5):1899
Abstract
The recent prospective isolation of a wide variety of somatically derived stem cells has affirmed the notion that homeostatic maintenance of most tissues and organs is mediated by tissue-specific stem and progenitor cells and fueled enthusiasm for the use of such cells in strategies aimed at repairing or replacing damaged, diseased, or genetically deficient tissues and organs. Hematopoietic stem cells (HSCs) are arguably the most well-characterized tissue-specific stem cell, with decades of basic research and clinical application providing not only a profound understanding of the principles of stem cell biology, but also of its potential pitfalls. It is our belief that emerging stem cell fields can benefit greatly from an understanding of the lessons learned from the study of HSCs. In this review we discuss some general concepts regarding stem cell biology learned from the study of HSCs with a highlight on recent work pertaining to emerging topics of interest for stem cell biology.
Figures
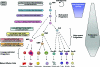
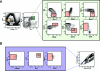
References
-
- Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. - PubMed
-
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. - PubMed
-
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. - PubMed
-
- Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

