Nitric oxide is an important mediator of renal tubular epithelial cell death in vitro and in murine experimental hydronephrosis
- PMID: 16877341
- PMCID: PMC1698789
- DOI: 10.2353/ajpath.2006.050964
Nitric oxide is an important mediator of renal tubular epithelial cell death in vitro and in murine experimental hydronephrosis
Abstract
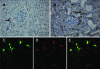
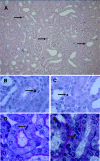
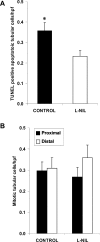
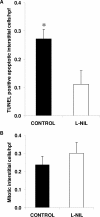
Macrophages play a pivotal role in tissue injury and fibrosis during renal inflammation. Although macrophages may induce apoptosis of renal tubular epithelial cells, the mechanisms involved are unclear. We used a microscopically quantifiable co-culture assay to dissect the cytotoxic interaction between murine bone marrow-derived macrophages and Madin-Darby canine kidney cells and primary murine renal tubular epithelial cells. The induction of tubular cell apoptosis by cytokine-activated macrophages was reduced by inhibitors of nitric oxide synthase whereas tubular cell proliferation was unaffected. Furthermore, cytokine-activated macrophages derived from mice targeted for the deletion of inducible nitric oxide synthase were noncytotoxic. We then examined the role of nitric oxide in vivo by inhibiting inducible nitric oxide synthase in the model of murine experimental hydronephrosis. l-N(6)-(1-iminoethyl)-lysine was administered in the drinking water between days 5 and 7 after ureteric obstruction. Macrophage infiltration was comparable between groups, but treatment significantly inhibited tubular cell apoptosis at day 7. Tubular cell proliferation was unaffected. Inducible nitric oxide synthase blockade also reduced interstitial cell apoptosis and increased collagen III deposition. These data indicate that nitric oxide is a key mediator of macrophage-directed tubular cell apoptosis in vitro and in vivo and also modulates tubulointerstitial fibrosis.
Figures




Similar articles
-
In vitro model of sepsis-induced renal epithelial reactive nitrogen species generation.Toxicol Sci. 2010 Jun;115(2):475-81. doi: 10.1093/toxsci/kfq058. Epub 2010 Feb 22. Toxicol Sci. 2010. PMID: 20176626 Free PMC article.
-
Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction.Kidney Int. 2000 Dec;58(6):2301-13. doi: 10.1046/j.1523-1755.2000.00414.x. Kidney Int. 2000. PMID: 11115064
-
Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells.Am J Pathol. 2010 Mar;176(3):1256-70. doi: 10.2353/ajpath.2010.090188. Epub 2010 Jan 14. Am J Pathol. 2010. PMID: 20075196 Free PMC article.
-
Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury.Kidney Int. 2002 Mar;61(3):862-71. doi: 10.1046/j.1523-1755.2002.00234.x. Kidney Int. 2002. PMID: 11849439
-
Renal tubular epithelial cells: the neglected mediator of tubulointerstitial fibrosis after injury.Cell Death Dis. 2018 Nov 13;9(11):1126. doi: 10.1038/s41419-018-1157-x. Cell Death Dis. 2018. PMID: 30425237 Free PMC article. Review.
Cited by
-
Macrophages in solid organ transplantation.Vasc Cell. 2014 Mar 11;6(1):5. doi: 10.1186/2045-824X-6-5. Vasc Cell. 2014. PMID: 24612731 Free PMC article.
-
Targeting c-fms kinase attenuates chronic aristolochic acid nephropathy in mice.Oncotarget. 2016 Mar 8;7(10):10841-56. doi: 10.18632/oncotarget.7460. Oncotarget. 2016. PMID: 26909597 Free PMC article.
-
Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis.Nat Med. 2016 Feb;22(2):202-9. doi: 10.1038/nm.4020. Epub 2016 Jan 11. Nat Med. 2016. PMID: 26752518 Free PMC article.
-
Indian Hedgehog release from TNF-activated renal epithelia drives local and remote organ fibrosis.Sci Transl Med. 2023 May 31;15(698):eabn0736. doi: 10.1126/scitranslmed.abn0736. Epub 2023 May 31. Sci Transl Med. 2023. PMID: 37256934 Free PMC article.
-
Synthesis, physicochemical characterisation and biological activity of anandamide/ɛ-polycaprolactone nanoparticles obtained by electrospraying.IET Nanobiotechnol. 2020 Feb;14(1):86-93. doi: 10.1049/iet-nbt.2019.0108. IET Nanobiotechnol. 2020. PMID: 31935683 Free PMC article.
References
-
- Gordon S. Macrophage-restricted molecules: role in differentiation and activation. Immunol Lett. 1999;65:5–8. - PubMed
-
- Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. - PubMed
-
- Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. - PubMed
-
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

