Integrin-mediated transforming growth factor-beta activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit
- PMID: 16877343
- PMCID: PMC1698780
- DOI: 10.2353/ajpath.2006.060049
Integrin-mediated transforming growth factor-beta activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit
Abstract
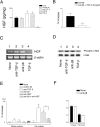
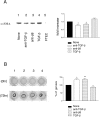
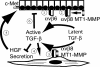
Trophic interactions between pulmonary epithelial and mesenchymal cell types, known as the epithelial-mesenchymal trophic unit (EMTU), are crucial in lung development and lung disease. Transforming growth factor (TGF)-beta is a key factor in mediating these interactions, but it is expressed in a latent form that requires activation to be functional. Using intact fetal tracheal tissue and primary cultures of fetal tracheal epithelial cells and fibroblasts, we demonstrate that a subset of integrins, alpha(v)beta(6) and alpha(v)beta(8), are responsible for almost all of the TGF-beta activation in the EMTU. Both alpha(v)beta(8) and alpha(v)beta(6) contribute to fetal tracheal epithelial activation of TGF-beta, whereas only alpha(v)beta(8) contributes to fetal tracheal fibroblast activation of TGF-beta. Interestingly, fetal tracheal epithelial alpha(v)beta(8)-mediated TGF-beta activation can be enhanced by phorbol esters, likely because of the increased activity of MT1-MMP, an essential co-factor in alpha(v)beta(8)-mediated activation of TGF-beta. Autocrine alpha(v)beta(8)-mediated TGF-beta activation by fetal tracheal fibroblasts results in suppression of both transcription and secretion of hepatocyte growth factor, which is sufficient to affect phosphorylation of the airway epithelial hepatocyte growth factor receptor, c-Met, as well as airway epithelial proliferation in a co-culture model of the EMTU. These findings elucidate the function and complex regulation of integrin-mediated activation of TGF-beta within the EMTU.
Figures
References
-
- Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000;105:193–204. - PubMed
-
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. - PubMed
-
- Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol. 2002;283:L510–L517. - PubMed
-
- Knight DA, Lane CL, Stick SM. Does aberrant activation of the epithelial-mesenchymal trophic unit play a key role in asthma or is it an unimportant sideshow? Curr Opin Pharmacol. 2004;4:251–256. - PubMed
-
- Holgate ST. Epithelial damage and response. Clin Exp Allergy. 2000;30(Suppl 1):37–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

