Sepsis and pathophysiology of anthrax in a nonhuman primate model
- PMID: 16877346
- PMCID: PMC1698797
- DOI: 10.2353/ajpath.2006.051330
Sepsis and pathophysiology of anthrax in a nonhuman primate model
Abstract
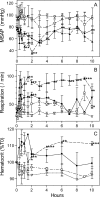
Studies that define natural responses to bacterial sepsis assumed new relevance after the lethal bioterrorist attacks with Bacillus anthracis (anthrax), a spore-forming, toxigenic gram-positive bacillus. Considerable effort has focused on identifying adjunctive therapeutics and vaccines to prevent future deaths, but translation of promising compounds into the clinical setting necessitates an animal model that recapitulates responses observed in humans. Here we describe a nonhuman primate (Papio c. cynocephalus) model of B. anthracis infection using infusion of toxigenic B. anthracis Sterne 34F2 bacteria (5 x 10(5) to 6.5 x 10(9) CFU/kg). Similar to that seen in human patients, we observed changes in vascular permeability, disseminated intravascular coagulation, and systemic inflammation. The lung was a primary target organ with serosanguinous pleural effusions, intra-alveolar edema, and hemorrhagic lesions. This animal model reveals that a fatal outcome is dominated by the host septic response, thereby providing important insights into approaches for treatment and prevention of anthrax in humans.
Figures







Similar articles
-
Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates.Shock. 2013 Jan;39(1):55-62. doi: 10.1097/SHK.0b013e318276f4ca. Shock. 2013. PMID: 23247122 Free PMC article.
-
Bacillus anthracis cell wall peptidoglycan but not lethal or edema toxins produces changes consistent with disseminated intravascular coagulation in a rat model.J Infect Dis. 2013 Sep;208(6):978-89. doi: 10.1093/infdis/jit247. Epub 2013 Jun 3. J Infect Dis. 2013. PMID: 23737601 Free PMC article.
-
The sepsis model: an emerging hypothesis for the lethality of inhalation anthrax.J Cell Mol Med. 2013 Jul;17(7):914-20. doi: 10.1111/jcmm.12075. Epub 2013 Jun 7. J Cell Mol Med. 2013. PMID: 23742651 Free PMC article.
-
Anthrax lethal and edema toxins in anthrax pathogenesis.Trends Microbiol. 2014 Jun;22(6):317-25. doi: 10.1016/j.tim.2014.02.012. Epub 2014 Mar 27. Trends Microbiol. 2014. PMID: 24684968 Free PMC article. Review.
-
Molecular determinants for a cardiovascular collapse in anthrax.Front Biosci (Elite Ed). 2014 Jan 1;6(1):139-47. doi: 10.2741/e697. Front Biosci (Elite Ed). 2014. PMID: 24389148 Free PMC article. Review.
Cited by
-
Anthrolysin O and fermentation products mediate the toxicity of Bacillus anthracis to lung epithelial cells under microaerobic conditions.FEMS Immunol Med Microbiol. 2011 Feb;61(1):15-27. doi: 10.1111/j.1574-695X.2010.00740.x. Epub 2010 Oct 14. FEMS Immunol Med Microbiol. 2011. PMID: 20946354 Free PMC article.
-
Nitric oxide as a regulator of B. anthracis pathogenicity.Front Microbiol. 2015 Sep 2;6:921. doi: 10.3389/fmicb.2015.00921. eCollection 2015. Front Microbiol. 2015. PMID: 26388860 Free PMC article.
-
A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models.Toxins (Basel). 2021 Jan 13;13(1):53. doi: 10.3390/toxins13010053. Toxins (Basel). 2021. PMID: 33450877 Free PMC article. Review.
-
Bioinspired detoxification of blood: The efficient removal of anthrax toxin protective antigen using an extracorporeal macroporous adsorbent device.Sci Rep. 2018 May 14;8(1):7518. doi: 10.1038/s41598-018-25678-0. Sci Rep. 2018. PMID: 29760471 Free PMC article.
-
Bacillus anthracis peptidoglycan stimulates an inflammatory response in monocytes through the p38 mitogen-activated protein kinase pathway.PLoS One. 2008;3(11):e3706. doi: 10.1371/journal.pone.0003706. Epub 2008 Nov 12. PLoS One. 2008. PMID: 19002259 Free PMC article.
References
-
- Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933–944. - PMC - PubMed
-
- Freedman A, Afonja O, Chang MW, Mostashari F, Blaser M, Perez-Perez G, Lazarus H, Schacht R, Guttenberg J, Traister M, Borkowsky W. Cutaneous anthrax associated with microangiopathic hemolytic anemia and coagulopathy in a 7-month-old infant. JAMA. 2002;287:869–874. - PubMed
-
- Borio L, Frank D, Mani V, Chiriboga C, Pollanen M, Ripple M, Ali S, DiAngelo C, Lee J, Arden J, Titus J, Fowler D, O’Toole T, Masur H, Bartlett J, Inglesby T. Death due to bioterrorism-related inhalational anthrax: report of 2 patients. JAMA. 2001;286:2554–2559. - PubMed
-
- Barakat LA, Quentzel HL, Jernigan JA, Kirschke DL, Griffith K, Spear SM, Kelley K, Barden D, Mayo D, Stephens DS, Popovic T, Marston C, Zaki SR, Guarner J, Shieh WJ, Carver HW, Meyer RF, Swerdlow DL, Mast EE, Hadler JL. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA. 2002;287:863–868. - PubMed
-
- Mina B, Dym JP, Kuepper F, Tso R, Arrastia C, Kaplounova I, Faraj H, Kwapniewski A, Krol CM, Grosser M, Glick J, Fochios S, Remolina A, Vasovic L, Moses J, Robin T, DeVita M, Tapper ML. Fatal inhalational anthrax with unknown source of exposure in a 61-year-old woman in New York City. JAMA. 2002;287:858–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

